Scientists at Scripps Research Institute have discovered sensory neurons near the spine that carry messages from fat or adipose tissue to the brain. This discovery overthrows the prevailing notion that circulating hormones are the sole messengers between adipose and brain cells. The findings were reported in the journal Nature (“The role of somatosensory innervation of adipose tissues“).
Nobel laureate, Howard Hughes Medical Institute investigator, neuroscience professor at Scripps, and co-senior author of the study, Ardem Patapoutian, PhD, said, “This is yet another example of how important sensory neurons are to health and disease in the human body.”
Li Ye, PhD, an associate professor of neuroscience, chair in chemistry and chemical biology at Scripps, and a senior author of the study said, “The discovery of these neurons suggests for the first time that your brain is actively surveying your fat, rather than just passively receiving messages about it. The implications of this finding are profound.”

In addition to housing long-term stores of energy and releasing it when needed, adipose tissue regulates hormones and signaling molecules that update the brain on satiety and metabolism. Disruption of these crucial functions of adipose tissue, causes or contributes to several metabolic diseases including diabetes, fatty liver disease, atherosclerosis, and obesity.
Previously, scientists suspected nerves extending into adipose tissues were part of the autonomic sympathetic nervous system that turn on fat-burning metabolic pathways during physical activity or stress. Lack of suitable research techniques for studying adipose tissue innervation, has until now prevented researchers from identifying the nature and function of neurons in fat cells. Conventional methods used to visualize or stimulate neurons in the brain or near the body surface fail to work in fat cells.
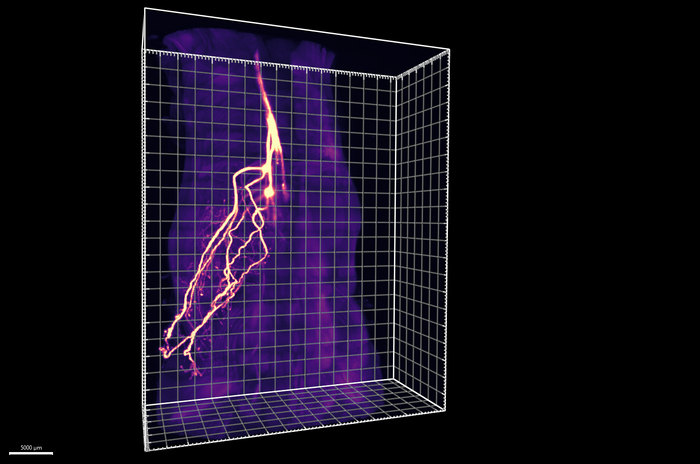
A tissue-clearing technique developed by Ye and his colleagues called HYBRiD, uses solvents to dissolve opaque biomolecules such as fat to render tissues transparent. In the current study, Ye’s team applied this protocol to track neurons that infiltrate deep into adipose tissue. They found nearly 50% did not relay to the sympathetic ganglia that flank the spinal cord but to the dorsal root ganglia (DRG), a cluster of cell bodies of sensory neurons. The technique enabled the team to visualize the entire axonal DRG projection from the cell body (soma) to the fat cells under the skin (subcutaneous adipocytes) and establish their anatomical origins.
Next, to assess the function of these DRG neurons in adipose tissue, Ye’s team used a genetic technique developed by the team earlier called ROOT (retrograde vector optimized for organ tracing). This allowed them to measure the consequences of removing selected small sets of sensory neurons within adipose tissue.
First author of the study, Yu Wang, a graduate student in both the Ye and Patapoutian labs said, “This research was really made possible by the way these new methods came together. When we first started this project, there weren’t existing tools to answer these questions.”
The investigators uncovered that experimentally removing sensory inputs from adipose tissue to the brain in mice triggers the generation of fat (lipogenesis) and increases the conversion of white fat to brown fat, resulting in enlarged fat pads under the skin and elevated body temperatures under normal ambient temperatures. High levels of brown fat are known to break down other fat and sugar molecules to produce heat.
These results suggest sensory and sympathetic innervation in adipose tissue may play opposing roles—whereas sympathetic neurons turn on fat burning, sensory neurons trigger fat production. Ye said, “There’s not just a one-size-fits-all instruction that the brain sends adipose tissue. It’s more nuanced than that. These two types of neurons are acting like a gas pedal and a brake for burning fat.”
Among questions that remain unanswered, is the nature of the messages to and from adipose tissues. Ye’s team is currently taking a closer look at the sensory cues in fat cells and searching for similar sensory neurons in visceral organs.