The “head them off at the pass” cliché endures for a good reason. It describes a hopeful scenario: the stalling or blocking of an unwelcome development at a strategic point. It is certainly the scenario favored by developers of inflammatory disease drugs. These developers would rather block a discrete step in a multistep inflammatory process—the equivalent of blocking a narrow mountain pass—than resort to a wider suppression of the immune system.
Unfortunately, conventional inflammatory disease drugs—the familiar steroidal and nonsteroidal drugs—frequently alter biological functions with system-wide effects. Such drugs give rise to adverse drug reactions. Also, when such drugs suppress immunity broadly, they weaken patients against infections.
Besides steroidal and nonsteroidal anti-inflammatories, there are now small-molecule drugs (such as Janus kinase inhibitors) and biologics (such as monoclonal antibodies against pro-inflammatory molecules). These medications are proving valuable in a range of applications, but there’s still room for improvement.
To stage even more targeted deployments, drug developers are exploiting the latest research about inflammatory pathways. Some developers are excited that disparate inflammatory conditions appear to share pathways and present the same targets. Conceivably, well-targeted drugs could have multiple indications. However, inflammatory pathways are intricate. Indeed, they can activate and resolve inflammatory processes in varied ways in different tissues and diseases. Still, drug developers are showing that if they can get the lay of the land, they can head inflammation off at the pass.
A wider reach and a tighter grasp
At IGM Biosciences, Mary Beth Harler, MD, is president of IGM autoimmunity and inflammation, a business unit tasked with realizing the potential of the company’s IgM antibody technology platform. Essentially, Harler’s unit is combining the strengths of IgM antibodies and IgG antibodies to inactivate pro-inflammatory cytokines and dampen inflammatory signals more effectively.
When faced with a pathogen, the immune system first produces nonspecific (but potent) IgM antibodies, and then, later, highly pathogen-specific IgG antibodies. The specificity of IgG is a major strength. It has already been harnessed by several drug developers. For example, AbbVie developed adalimumab (Humira), a recombinant monoclonal IgG antibody, to neutralize the inflammatory cytokine tumor necrosis factor alpha (TNF-a). The drug is used to treat arthritis, Crohn’s disease, ulcerative colitis, and psoriasis.
IgG antibodies, however, have just two antigen-binding sites, whereas IgM antibodies have ten. To make the most of IgG antibody specificity and IgM antibody potency, IGM Biosciences tried an unusual approach. “We graphed highly specific IgG binding sites onto an IgM molecule,” Harler says. “It equates to the difference between trying to pick something up with two fingers versus [ten fingers].”
More sites to bind ligands means more inflammatory cytokines neutralized per antibody—and not just cytokines like TNF-a. “I see a very rich field of validated targets that we can strongly consider,” Harler declares. She adds that pentameric antibodies could be produced that would be capable of neutralizing targets “that, frankly, were not accessible through more conventional IgG-based therapy.”
IGM Biosciences recently announced that its technology attracted an enthusiastic development partner. This partner, Sanofi, will pay IGM Biosciences $150 million upfront to collaborate in the creation, development, manufacture, and commercialization of IgM antibody agonists against three oncology targets and three immunology/inflammation targets. IGM Biosciences is eligible to receive potentially over $6 billion in aggregate development, regulatory, and commercial milestones.
Although the commercial potential of IgM antibodies is exciting, Harler has additional motivations. For example, she is motivated by scientific curiosity. She remarks, “The wealth of knowledge [about inflammatory pathways] that has been generated over the past two to three decades is breathtaking.” Diseases that may differ dramatically from one another, and even from patient to patient, often share the same inflammatory pathways, and they often present similar targets for potential therapies.
“If there is an organizing principle, it’s around the pathways,” Harler continues. “Autoimmune disease is almost system agnostic. It’s more about the pathways that may manifest in certain organ systems.”
Another motivation, Harler confides, is more personal. “I have a daughter who was diagnosed with ulcerative colitis at age 10,” she says. “The risk of a colectomy and a colostomy by age 20 for such a patient as my daughter is unacceptably high.”
Similar sentiments are no doubt widely shared, given that inflammation accompanies may chronic diseases. The Rand Corporation estimates that in 2014, nearly 60% of Americans had at least one chronic condition, 42% had more than one, and 12% of adults had five or more. According to the World Health Organization, three out of five people across the globe die due to chronic inflammatory diseases such as stroke, chronic respiratory diseases, heart disorders, cancer, obesity, and diabetes.
More than a mere framework
Engitix, a biotech firm based in London, focuses on how long-term chronic inflammation leads to the scarring and rigidity of tissue fibrosis. The company maintains fibrosis programs (sclerosing cholangitis, nonalcoholic steatohepatitis, and intestinal fibrostenosis in inflammatory bowel disease [IBD]) as well as solid tumor programs (pancreatic adenocarcinoma and hepatocellular carcinoma).
Despite the success of TNF-a-targeting drugs like adalimumab, “a significant number of patients with IBD, unfortunately, progress toward the most advanced or more serious complication of IBD, which is fibrostenotic disease,” says Giuseppe Mazza, PhD, the co-founder and CEO of Engitix. Intestinal fibrosis can lead to a narrowing of the intestines only addressable through surgery—no effective drugs currently exist.
To understand why some IBD patients progress to fibrosis, Engitix has turned its attention to the extracellular matrix (ECM), the network of collagen and other proteins that help structure tissues. “People have usually thought of the ECM as a scaffold, a bystander product, that keeps our tissue together,” Mazza says. “What we have discovered, together with, of course, other groups around the world, is that the ECM is a highly bioactive environment.”
According to Mazza, fibrotic disease caused by long-term chronic inflammation alters the ECM and stiffens tissues. He notes that the ECM also “plays a key role in driving the disease forward.”
Now in partnership with Takeda, Entigix is using its technology to study the ECM in healthy patients and in patients with IBD to identify both biomarkers and potential therapeutic targets.
“If we can understand this, we can combine anti-inflammatory therapy together with direct antifibrotic therapy to reverse or stabilize the disease,” Mazza states. The combination approach that Mazza has in mind incorporates existing therapies and ECM-based therapies. Some sort of combination approach will be necessary, he explains, because “complex diseases are not going to be treated by a single therapy.”
Disregarding DAMPs
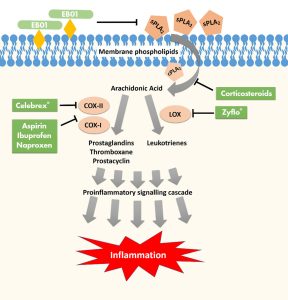
Edesa Biotech of Markham, Canada, has two drug candidates in later stage development. The first candidate is EB01, an inhibitor of secretory phospholipase A2. It is being evaluated as a topical treatment for chronic allergic contact dermatitis (ACD). ACD is a common, potentially debilitating condition and occupational illness.
The second candidate is EB05, a monoclonal antibody therapy that is being evaluated as a treatment for acute respiratory distress syndrome (ARDS). In ARDS, runaway inflammation leads to fluid filling the lungs. There are currently no drugs to treat the condition, and patients are typically supported with oxygen, with those who survive often dealing with fibrotic scarring of their lungs.
Initially, Edesa’s EB05 work focused on ARDS driven by influenza or chemical inhalation. Later, when COVID-19 emerged, Edesa recognized that the ARDS mechanism it uncovered also applied to COVID-19-driven ARDS. “It was obvious,” recalls Michael Brooks, PhD, president of Edesa, “that if there was good evidence for this mechanism in ARDS, then COVID-19 shouldn’t really be any different than influenza or chemical inhalation.”
Edesa found that the mechanism behind ARDS involved toll-like receptor 4 (TLR4), a signaling receptor that is widely expressed on immune cells. When TLR4 recognizes a foreign body, it triggers an inflammatory signaling cascade to clear out the pathogen. The problem, Brooks notes, is that TLR4 receptors also detect molecular motifs that result from cell death or tissue injury. These motifs are called damage-associated molecular patterns (DAMPS).
“There is a place that is sort of a point of no return,” Brooks remarks. “The damage starts to become the problem itself.” In other words, inflammation can cause damage that causes more inflammation.
Edesa’s EB05 aims to break the feedback loop in an unusual way. Instead of competing with DAMPS to prevent them from binding with TLR4, EB05 blocks the receptor’s dimerization, and hence its activation, regardless of DAMP binding.
Brooks asserts that EB05 “blocks activation of the receptor despite the amount of damage that’s surrounding the cell independent of the amount of extracellular signal.”
Edesa reported positive initial results from a Phase II/III clinical trial of EB05 in COVID-19 ARDS patients in September 2021, which showed a greater than 68% reduction in risk of death compared with placebo over 28 days. Edesa is still recruiting patients and shifting into a Phase III confirmatory trial with EB05.
A gene expression profile test
Besides driving therapeutic development, molecular pathway knowledge can guide therapeutic selection. For example, Castle Biosciences is developing a gene expression profile (GEP) test to predict the response to systemic therapy in patients with moderate-to-severe psoriasis, atopic dermatitis, and related inflammatory skin conditions. It’s an approach that could bring greater efficiency to a process that is too often reactive rather than proactive, says Robert Cook, PhD, Castle’s senior vice president of research and development.
“Trial and error has been the primary way that clinicians are trying to implement these therapies,” he says. “For atopic dermatitis, Dupixent (dupilumab) is a newer therapy that has been very effective, but it’s not effective for everyone. A lot of clinicians may focus on Dupixent at treatment outset, but they learn later on that they need to switch gears.”
In the United States, about 18 million patients receive atopic dermatitis and psoriasis diagnoses each year. A subset of these patients—about 450,000 patients—have moderate to severe disease and are eligible for systemic therapies. These are the patients Castle hopes to help.
In April, Castle announced that its GEP test was the subject of a new poster paper. The paper described how samples were collected via a noninvasive skin scraping technique and evaluated via the quantitative real-time polymerase chain reaction technique. The paper also reported that the test was able to process sufficient RNA to reproducibly assess gene expression.
Castle hopes to identify biomarkers that could be used to guide treatment decisions. According to Cook, the company has launched an observational trial aiming to enroll 4,800 patients with atopic dermatitis or psoriasis across 52 participating centers. More than 140 patients have been enrolled so far.
“With those patient samples, we can begin expanding the discovery process to identify the relevant biomarkers,” he declares. “We expect to launch the test by the end of 2025.”
Restoring immune homeostasis
Atopic dermatitis is also important to Brickell Biosciences. Although the condition isn’t a primary target for the company’s new lead drug candidate, a DYRK1a inhibitor, it presents an excellent opportunity for the company to test its technology.
“We chose atopic dermatitis, not because we necessarily want to enter that space, but because the condition affects a large patient population and causes visible inflammation,” says Monica Luchi, MD, a rheumatologist and chief medical officer at Brickell. “You can see it on the skin.”
Therapies based on DYRK1a could potentially treat much more than skin inflammation—including diseases such as rheumatoid arthritis, type 1 diabetes, and Alzheimer’s.
DYRK1a—dual-specificity tyrosine-phosphorylation-regulated kinase 1A—is an enzyme with two important roles in inflammatory processes. In the innate immune system, the inhibition of DYRK1a prevents TLR4 pathway signaling that releases pro-inflammatory cytokines. In the adaptive immune system, inhibition of DYRK1a can restore immune homeostasis between regulatory and pro-inflammatory T cells. Without sufficient or effective regulatory T cells, Luchi relates, “the pro-inflammatory cells can actually kind of run amok.”
Brickell has several DYRK1A inhibitors in development, the most advanced of which, BBI-02, will enter a first-in-human Phase Ib clinical trial before summer’s over, Luchi notes. BBI-02 could treat atopic dermatitis, rheumatoid arthritis, and type 1 diabetes. Another DYRK1a inhibitor, a “next-generation kinase inhibitor” that Brickell hopes will treat neuroinflammatory diseases such as Alzheimer’s disease and Pick’s disease, is still in the experimental characterization phase.
The trick to taking these new therapies from bench to bedside, Luchi remarks, is to understand that while different diseases may share inflammatory pathways and even targets such as DYRK1a, the specific ways inflammation manifests in each condition—and even each patient—can vary dramatically.
“We thought that we understood all these diseases by subdividing them,” she observes. “We’re learning that even within the subdivisions, there are other diseases.”

