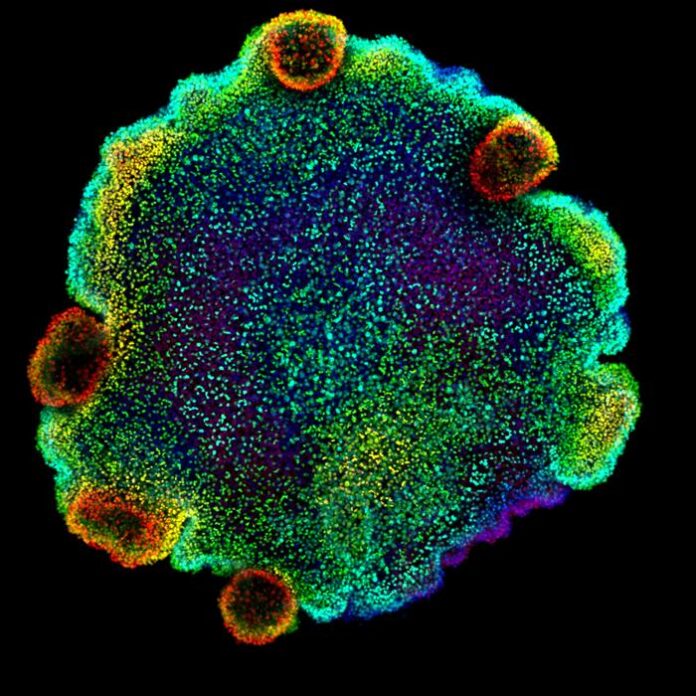
The results of research in tiny marine animals called placozoans have given scientists new insights into the possible evolution of neurons. A team headed by scientists at the Barcelona Institute of Science and Technology Centre for Genomic Regulation, studying the handful of different cell types in these ancient, millimeter-sized marine organisms, uncovered evidence that the animals’ specialized secretory cells may have given rise to neurons in more complex organisms. Reporting on their study in Cell, the researchers and international collaborators say their approach, using techniques including comparative single-cell genomics, phylogenetics and chromatin profiling, should help researchers better understand how different cell types originate and evolve.
“Placozoans lack neurons, but we’ve now found striking molecular similarities with our neural cells,” said Xavier Grau-Bové, PhD, a postdoctoral researcher at the Centre for Genomic Regulation. Grau-Bové is co-first author of the team’s published report, which is titled “Stepwise emergence of the neuronal gene expression program in early animal evolution.”
Placozoans are tiny animals, around the size of a large grain of sand, which graze on algae and microbes living on the surface of rocks and other substrates found in shallow, warm seas. These simple sea creatures live without any body parts or organs, and are thought to have first appeared on Earth around 800 million years ago. They are one of the five main lineages of animals, alongside Ctenophora (comb jellies), Porifera (sponges), Cnidaria (corals, sea anemones and jellyfish) and Bilateria (all other animals).
Placozoans have a body plan that consists of two cell layers, and a few different cell types. The animals coordinate their behavior thanks to peptidergic cells, special types of cells that release small peptides which can direct movement or feeding. “These small disc-shaped animals not only have nine morphologically described cell types and no neurons but also show coordinated behaviors triggered by peptide-secreting cells,” the team noted. “The collective behavior of placozoan cells is controlled by paracrine signaling, via small neuropeptides (NPs) secreted by low-abundance peptidergic cells that lack cellular projections and synapses.”
Driven by the intrigue of the origin of these cells, the researchers employed an array of molecular techniques and computational models to understand how placozoan cell types evolved and pieced together how our ancient ancestors might have looked and functioned.
The researchers first made a map of all the different placozoan cell types, annotating their characteristics across different species. ”To understand the cellular diversity in placozoans, here, we used single-cell transcriptomics to characterize cell type gene expression and cell differentiation dynamics across four species,” they wrote. “To systematically characterize and compare placozoan cell types, we sampled over 65,000 single-cell transcriptomes from four different placozoans.” Each cell type has a specialized role which comes from certain sets of genes. The resulting expression maps, or cell atlases, allowed the researchers to chart clusters or ‘modules’ of these genes. They then created a map of the regulatory regions in DNA that control these gene modules, revealing a clear picture about what each cell does and how they work together. “We combined these expression maps with genome-wide profiling of cis-regulatory elements (REs) to decode regulatory programs in placozoans.” Finally, they carried out cross-species comparisons to reconstruct how the cell types evolved. “… we conducted cross-species comparative analyses to reconstruct the evolution of placozoan gene modules and, ultimately, the emergence of the neuronal gene expression program,” they further wrote.
The research showed that the main nine cell types in placozoans appear to be connected by many “in-between” cell types which change from one type to another. “… we detected the presence of metacells with intermediate expression profiles between cell types, which we termed ‘‘intermediate’’ cells,” the investigators wrote. “Intermediate cells lack specific gene markers, and they only expressed a small subset of the genes expressed in each of the terminal cell types …”
The cells grow and divide, maintaining the delicate balance of cell types required for the animal to move and eat. The researchers also found fourteen different types of peptidergic cells, but these were different to all other cells, showing no in-between types or any signs of growth or division. “In all four cell atlases, we identified a high diversity of peptidergic cells,” they commented. “The cross-species analysis allowed us to group them into fourteen types …”. Surprisingly, the peptidergic cells shared many similarities to neurons – a cell type which didn’t appear until many millions of years later in more advanced animals such as cnidaria and bilateria. “Overall, the molecular signatures identified in peptidergic progenitors are intriguingly similar to those in neuronal progenitors in cnidarians and bilaterians,” the team noted. “These cells not only do not show signatures of cell cycle and intermediate states with other somatic cells, but appear to derive from a distinct progenitor cell population with multiple molecular signatures typically associated with neurogenesis in cnidarians and bilaterians.” Cross-species analyses revealed these similarities were unique to placozoans and do not appear in other early-branching animals such as sponges or comb jellies (ctenophores).
The similarities between peptidergic cells and neurons were threefold. First, the researchers found that these placozoan cells differentiate from a population of progenitor epithelial cells via developmental signals that resemble neurogenesis, the process by which new neurons are formed, in cnidaria and bilateria. Second, they found that peptidergic cells have many gene modules required to build the part of a neuron which can send out a message (the pre-synaptic scaffold). However, these cells are far from being a true neuron, as they lack the components for the receiving end of a neuronal message (post-synaptic) or the components required for conducting electrical signals.
Finally, the authors used deep learning techniques to show that placozoan cell types communicate with each other using a system in cells where specific G-protein coupled receptors (GPCRs) detect outside signals and start a series of reactions inside the cell. These outside signals are mediated by neuropeptides (NPs), chemical messengers used by neurons in many different physiological processes. “Peptidergic cells share other similarities with cnidarian/bilaterian neurons,” they noted. “For example, they express large numbers of GPCRs (although very few ion channels) and unique combinations of post-translationally modified NPs …”.
“We were astounded by the parallels,” says Sebastián R. Najle, PhD, co-first author of the study and postdoctoral researcher at the Centre for Genomic Regulation. “The placozoan peptidergic cells have many similarities to primitive neuronal cells, even if they aren’t quite there yet. It’s like looking at an evolutionary stepping stone.”
The study demonstrates that the building blocks of the neuron were forming 800 million years ago in ancestral animals grazing inconspicuously in the shallow seas of ancient Earth. From an evolutionary point of view, early neurons might have started as something like the peptidergic secretory cells of today’s placozoans. “In terms of the evolutionary emergence of nervous systems, our findings indicate that key neuronal functional and ontogenetic gene modules originated in the context of non-neuronal secretory cell type networks, as proposed by the chemical brain hypothesis,” the team suggested. These cells communicated using neuropeptides, but eventually gained new gene modules which enabled cells to create post-synaptic scaffolds, form axons and dendrites and create ion channels that generate fast electrical signals—innovations which were critical for the dawn of the neuron around one hundred million years after the ancestors of placozoans first appeared on Earth.
The complete evolutionary story of nerve systems has still yet to be told. The first modern neuron is thought to have originated in the common ancestor of cnidarians and bilaterians around 650 million years ago. And yet, neuronal-like cells exist in ctenophores, although they have important structural differences and lack the expression of most genes found in modern neurons. The presence of some of these neuronal genes in the cells of placozoans and their absence in ctenophores raises fresh questions about the evolutionary trajectory of neurons. “How ctenophore neurons fit in this scenario remains a major question as, despite having a largely peptidergic nervous system, they lack the conserved expression of the specific neuronal machinery and neurogenic program we report here for placozoans,” the investigators stated. “Ctenophores have neural nets, with key differences and similarities with our own,” Grau-Bové noted. “Did neurons evolve once and then diverge, or more than once, in parallel? Are they a mosaic, where each piece has a different origin? These are open questions that remain to be addressed.”
The authors believe that, as researchers around the world continue to sequence high-quality genomes from diverse species, the origins of neurons and the evolution of other cell types will become increasingly clear. “Cells are the fundamental units of life, so understanding how they come into being or change over time is key to explain the evolutionary story of life. Placozoans, ctenophores, sponges and other non-traditional model animals harbor secrets that we are only just beginning to unlock,” concluded ICREA research professor Arnau Sebé-Pedros, PhD, corresponding author of the study and junior group leader at the Centre for Genomic Regulation.
Noting limitations of their study, the authors concluded, “… our study exemplifies how dense phylogenetic sampling of cell atlases will enable ever-more detailed reconstruction of ancestral cell states and cellular innovations … In the future, this systematic genotype-cellular phenotype mapping should help us better understand how cell type programs originate and evolve.”