In mass spectrometry (MS), workflows are being streamlined and analyses are becoming more sophisticated. In short, MS is becoming faster, easier to use, and more functional. This is welcome news for proteomics and metabolomics researchers.
As upstream workflows are automated and as barriers to interpretation fall, MS becomes accessible even for smaller laboratories. Instruments are being designed for high-throughput analysis and enhanced sensitivity.
Interpreting the data
“There’s a high learning curve associated with MS,” says Gary Patti, PhD, chief scientific officer, Panome Bio. “The equipment is complicated.” With MS, workflow management and data interpretation are equally complicated. Panome Bio is working to simplify these tasks and thus make MS feasible for more researchers.
“Interpretation,” Patti explains, “is the biggest limiting factor for most people. For example, if you take one drop of blood and analyze it on a mass spectrometer, you’ll see some 20,000 to 30,000 different peaks. As scientists, we have to figure out how to annotate and quantify them, map them to pathways and reactions, and so on.”
Many of those peaks, however, don’t correspond to anything meaningful. Panome Bio is streamlining interpretation by eliminating the peaks that aren’t associated with the sample, such as impurities in the solvents.
Peak elimination was addressed a decade ago by the scientists who eventually became associated with Panome Bio. By feeding cells specific nutrients that were encoded with labels, the scientists gave themselves a way to distinguish between peaks that corresponded to chemical entities and peaks that merely reflected noise.
The approach was applied to a wide range of samples and cells, and the results were entered into a continuously expanding database. Now, when Panome Bio runs samples on behalf of a client, the database provides a background for machine learning–driven assessments. In these assessments, only the peaks associated with the samples are identified.
“This is a huge simplification,” Patti asserts. After eliminating everything not associated with their samples, the application can, Patti continues, “assign names and quantification levels to those that remain.” This makes MS technology accessible to those without the expertise to interpret the peaks.
Panome Bio offers solutions for proteins as well as metabolites, and the company continues to expand its databases. “Our platform is well established,” Patti declares. “We’re able to monitor a lot of biology in ways that previously were inaccessible using traditional tools.” The platform is available now as a service.
Electron capture dissociation
Zef Scientific is working with the University of Arizona and e-MSion (a company recently acquired by Agilent Technologies) to develop new applications that enhance protein characterization using liquid chromatography–mass spectrometry (LC-MS) with electron capture dissociation (ECD) technology. The goal is to further enable top-down, middle-down, and native workflows for more comprehensive characterization of protein sequences, post-translational modifications, and higher-order structures.
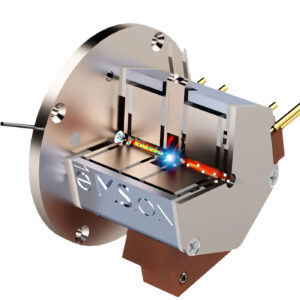
The technology developed by e-MSion is called the ExD cell. “Once installed, the ExD cell provides complimentary data to traditional collision-induced dissociation (CID)-based fragmentation, and it’s simple to use,” says Rick Carberry, business development and marketing manager, Zef Scientific.
The device brings new capabilities to the laboratory for charge reduction as well as native MS and top-down proteomics workflows. Carberry specifies that the device “performs electron-based fragmentation and takes multiple-charged ions and turns them—predominantly—into single-charged ions. This makes it much easier to interpret the data, especially in complex biological samples.”
Zef Scientific’s mission is to provide critical in-field service support for both the new ECD technology and the host LC-MS system. Researchers can update their existing quadrupole time-of-flight systems (such as those provided by Agilent or Waters) or orbitrap systems (provided by Thermo Fisher Scientific), and do so economically.

Initially, Zef Scientific installed the ECD technology on the Thermo Q-Exactive Ultra-High Mass Range MS instrument at the University of Arizona laboratory run by Michael Marty, PhD, assistant professor of chemistry and biochemistry. The technology is now being adopted by many leading laboratories in academia and industry. “They’re beginning to produce some fantastic results,” Carberry notes.
Sample prep automation
With improvements in liquid chromatography systems and separation columns, MS systems are becoming more accurate and expanding the utility of proteomics for larger-scale studies.
“Sample preparation is still, unfortunately, manual for a lot of laboratories,” admits Kristan Bahten, senior product marketing manager, Thermo Fisher Scientific. “Such approaches can be time consuming and are highly prone to errors that can compromise even the best LC-MS performance. Automation can address a lot of those issues by providing highly reproducible and confident results that can increase interlab and intralab reproducibility.”
Automating solutions throughout the proteomics workflow leads to greater standardization among laboratories, which allows results to be compared more easily.

Thermo Fisher introduced the AccelerOme system last year to standardize and simplify sample preparation for LC-MS analysis. It features pre-validated methods, kit-style reagents, and built-in UV measurement (to help researchers measure peptide concentrations before the LC-MS analysis), as well as a software wizard (to guide users through the process).
“Everything is integrated into the system,” says Bani Suri, PhD, product manager, laboratory automation, Thermo Fisher Scientific. The specifics of the experiment are entered, transferred throughout the system, and downloaded into the AccelerOme sample prep platform. There, it can automatically generate a sequence strings calendar for LC-MS analysis and transfer all that data to proteome discovery without reentry.
Now, Bahten says, “We are looking to add support for TMT18-plexing, increasing capacity up to a full 96-well plate and expanding the sample mass range beyond 10 µg, to 100 µg.” The company also plans to expand the list of applications to include fractionation and enrichment workflows.
Upstream workflow automation
“Automation hasn’t been implemented much in proteomics yet, but it is picking up,” says Nicolai Bache, PhD, head of applications, Evosep. He adds that the bottlenecks caused by slow processing speeds and sample variations must be reduced before proteomics applications can expand beyond the research laboratory and reach into diagnostic and clinical settings.
To enable that, Evosep and ReSyn Biosciences are collaborating to develop a standardized, end-to-end workflow that seamlessly connects liquid handlers and mass spectrometers. ReSyn Biosciences’ focus in all this is the optimization of magnetic beads. The aim is to create modular units that can be deployed as needed for specific workflows. “We can easily adapt variables in sample preparation, including the bead-to-sample ratio, and control interactions through mixing speeds and times,” asserts Justin Jordaan, PhD, CEO and CSO of ReSyn Biosciences.
“Magnetic beads are easily automated on liquid handling stations by the simple inclusion of a magnetic rack or deck,” Jordaan continues. “Combined with our proprietary bead technology, this allows us to miniaturize, optimize, and automate routine workflows in proteomics, with the aim of standardizing these protocols for the robust analysis of samples in proteomics.”
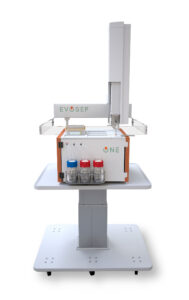
The teams are working to optimize the workflow of the Evosep One, a standardized LC solution that can run 100 samples per day, with 0.9 seconds run to run. It lets users change columns while measuring a sample set and shows “good reproducibility between instruments,” notes Dorte Bekker-Jensen, PhD, senior scientist, Evosep.
That device integrates offline peptide loading and desalting, integrating elution with liquid chromatography. Thus, the use of Evotips (disposable trap columns) eliminates the sample handling steps, improves storage before analysis, and lowers injection cycle overheads. It may be possible to integrate peptide loading with sample enrichment or other workflows.
Evosep is working to integrate the Opentrons OT-2 liquid handler into the workflow to handle loading and automate protein aggregation capture (PAC) digestion using magnetic beads. It is an alternative to Thermo Fisher Scientific’s large Kingfisher Flex robot.
The Opentrons OT-2 is not ready for high throughput, though. Currently, it can process only 8 samples in parallel, versus Kingfisher’s 96. However, according to Evosep, it is versatile, affordable, and capable of handling custom labware. Currently, the company is working to integrate Evotip loading and transfer the PAC workflow to the Opentrons OT-2.
“The Evosep loading module is not yet commercially available,” Bache stresses, but internal experiments indicate that transferring workflow to Opentrons is promising. For example, researchers digested 50 µg of HeLa cells in eight technical replicates to measure miscleavages and to reduce digestion time.
“Our default workflow contains five washes on the Kingfisher,” Bekker-Jensen says. Researchers were able to reduce the number of washes to as few as one while fully cleaving an average of 88% of the peptides before the digestion. According to Bekker-Jensen, the researchers found “that digestion at 37°C was best, that digestion at room temperature overnight provides similar results, and that digestion at room temperature for four hours gives acceptable results.” Ideally, peptides should be loaded as soon as the digestion is quenched.
Automation is making it easier for laboratories of many sizes to use LC and MS. “The medium-term prospects are to enable these applications by developing robust and fully automated high-throughput solutions for analysis of the biological samples,” Jordaan points out. “This requires close collaboration between partners with unique technologies, to provide solutions that are sufficiently robust for enabling these clinical applications.”
To that end, Evosep recently launched Evosep Biolabs. Its mission is to be a center of excellence for high-throughput proteomics, to speed sample prep and analysis for MS (ideally by 10-fold), and to increase throughput 100-fold. Proteomics is driving a resurgence of interest in MS, and equipment manufacturers are responding rapidly with enhancements and new technologies to advance proteomics at multiple levels.