Hair loss can be distressing, even devastating, but it isn’t considered a serious health problem. Consequently, hair loss has been addressed by relatively few biotech companies. One of those companies is Stemson Therapeutics. It is pioneering the use of induced pluripotent stem cells (iPSCs) to regenerate hair. The company says that its iPSC-based approach is well positioned to meet the enormous market demand for solutions to scarring alopecia, androgenic alopecia, and chemotherapy-induced alopecia.

“When you are experiencing the degeneration of tissue that manifests as hair loss, the cells that are responsible for generating hair are dying,” says Geoff Hamilton, CEO of Stemson. “The logical approach is to replace or recapitulate those lost cells. That’s where stem cells come into play.”
Although some scientists have tried to cultivate adult follicle-derived cell populations to generate transplant material, that approach hasn’t been especially effective. Stemson has a different approach, one that relies on iPSCs. “They give us a better and more reliable, robust, and scalable way of generating the source population of the highly specialized cells that are needed to grow hair follicles,” Hamilton asserts.
Hamilton cautions that Stemson’s approach is currently in preclinical development and still needs to be tested in humans. So, development will continue several years before a treatment enters human trials, and a few years will pass before that treatment becomes available, assuming it succeeds. He adds, “The science we’re performing is so novel, none of us can predict exactly when we’ll be ready for trials.”
When trials do eventually begin, they will evaluate a first-generation, autologous treatment. “That’s simpler and less clinically risky,” Hamilton states. “We do have plans, long-term, to develop an allogeneic approach that would be more economical.
Procedural details
“Today’s hair transplant surgeries are a two-step procedure that begins with excising hair-containing donor tissue—typically 1-mm-sized hole punches,” Hamilton notes. “These hole punches are then inserted into equally large hole punch areas in the recipient.”
Stemson’s procedure skips the excision step and starts by extracting somatic cells (for example, blood or skin cells) from the patient and reprogramming them into iPSCs. The iPSCs are amplified and differentiated into hair follicle germs, which then are primed and prepared for transplantation to the patient.
“As the insertion points heal, the tissue vascularizes, which is vital for nourishing the hair germs,” Hamilton details. “The hair germs form into hair follicles, and those cells begin to proliferate, growing up from the transplant depth to reach the skin surface tissue.”
The process is rather like planting seeds in a garden, with one notable exception. In Stemson’s method, the pores must be held open during the healing process so the hair shaft can emerge through the skin. This is accomplished using very small biodegradable scaffolds—placeholders—that are similar to the material used for surgical stitches. “It takes a couple of months for the hair to emerge,” Hamilton remarks.
Androgenic alopecia occurs because the biochemical environment disrupts and damages the hair follicle tissue—particularly the dermal papilla component. However, that environment may not damage the newly transplanted hair germs. “Because the hair germs are neonatal-like,” Hamilton explains, “they are less susceptible to the biochemicals—dihydrotestosterone (DHT), for instance—that damage the dermal papilla in the hair follicle.” As the new hair follicle is formed, its clean, natural environment should offer some protection from those biochemicals.
Whether, or how long, that protection lasts in humans must be determined in clinical trials. To further improve the chances that hair will grow and that the hair follicles will remain healthy, Stemson plans to assess naturally resistant follicles to identify their key protective properties. The company may use those findings to guide hair germ selection.
Familial collaboration
Stemson was founded because a father and son wanted to work together on a project that combined their talents. Co-founder and CSO Alexey Terskikh, PhD, a scientist at the Sanford Burnham Prebys Medical Discovery Institute, has worked on stem cells and regenerative medicine for most of his career. His father is a biologist who works with skin. They decided to take advantage of an opportunity to collaborate on the generation of new dermal papilla cells.
“They were the first in the world to differentiate iPSCs into dermal papilla cells,” Hamilton points out. “Once those human dermal papilla cells generated hair follicles in mice, father and son realized that they had, potentially, an attractive commercial opportunity.” Hamilton joined Alexey Terskikh in 2017 to build Stemson.
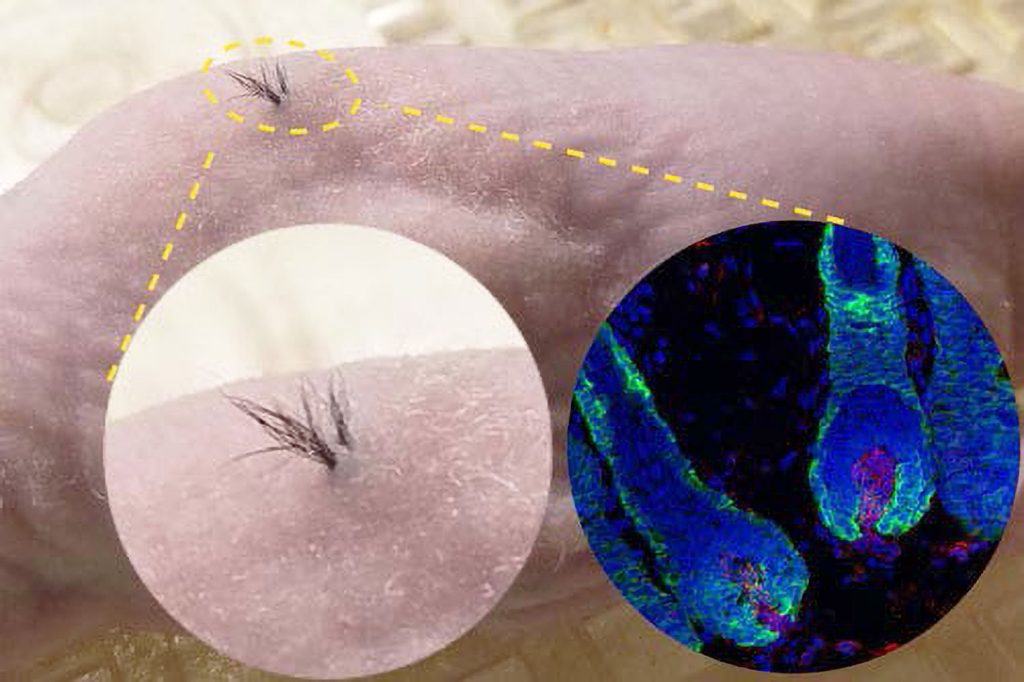
The company faced two key challenges in its early years: “One was an investment challenge,” Hamilton recalls. “There is an embarrassingly small amount of investment dollars in the industry invested in treatments for hair loss. It’s rarely a part of any company’s strategy.” Allergan, one of Stemson’s early investors, is the exception.
“The second challenge,” Hamilton continues, “was building a robust scientific team. Few people focus their biology work or careers around hair loss treatments.”
Scientific challenges
Hamilton says the company is past those hurdles today. Now the challenges are scientific. “The hair follicle is a very complex mini-organ, and it contains many cell types that work together,” he relates. “How you put those cell types together to drive hair follicle development and get the follicle to survive transplantation into a complex living body is challenging.” Stemson’s team is looking at the variables, controls, and timing issues, as well as strategies to manufacture the various follicular components.
The other difficulty, he says, is finding a suitable experimental model: “Most therapeutic development occurs in mice, but the phenomenon we observe in mice does not necessarily translate into humans.” The team anticipates that pigs will more accurately approximate the human skin environment.
If this approach succeeds in humans, it would be a complete breakthrough, enabling new hair to grow not just for a brief time, but indefinitely. “It would be the first-ever supply of de novo hair follicles,” Hamilton declares. He suggests that it would be possible even for people who are bald to undergo treatment and grow a full head of hair.
According to Hamilton, Minoxidil is a Band-Aid approach. “It temporarily excites the hair so some of the remaining stem cells grow into hair follicles, but it doesn’t prevent the long-term degeneration of the follicles,” he maintains. “Eventually, it will stop working because there are no longer enough follicular cells.” Propecia (finasteride), which often is used with minoxidil, tries to stop further hair loss, but it doesn’t prevent degradation.
Stemson’s iPSC technology appears to be scalable. That is, it may be able to generate as many hair follicles as each patient needs.
The company is focusing now on completing its R&D work. “We must demonstrate that our solution works consistently in pigs,” Hamilton says. Then, Stemson must attract additional funding to advance to clinical trials.