The recent approval by the U.S. Food and Drug Administration (FDA) of Casgevy, a therapy developed by Vertex Pharmaceuticals and CRISPR Therapeutics that uses CRISPR gene editing to treat patients with sickle cell disease, is a watershed moment in the history of this nascent field.
I have been involved in gene therapy research for decades. Like many of my peers, I am thrilled that with this incredible breakthrough, CRISPR may be, as one story in GEN noted, entering “prime time” for patient applications. The ability of CRISPR to make precise changes to human DNA that can instruct a gene to either stop doing something harmful or, in the case of sickle cell patients, start doing something beneficial—specifically, supply healthy red blood cells—is remarkable.
But I remain concerned that a surge of understandable optimism can be perilous. Unexpected immune reactions to a gene therapy that uses a weakened form of a common respiratory virus were implicated in the death of a clinical trial volunteer in the 1990s and again in a patient last year. CRISPR was used in this more recent case, although it did not appear to play a role in the patient’s death. And just this month, Verve Therapeutics paused a gene editing clinical trial for heart disease following a patient adverse event, although here the finger of blame appears to point to a specific non-viral delivery vector.
I believe the risks of the CRISPR-enabled sickle cell treatment were thoroughly evaluated and vigorously debated in the run-up to the approval of Casgevy last December. But I also know from first-hand experience that a fundamental risk of using CRISPR to modify genes in people is not getting the attention it deserves. That’s mainly because many researchers are not using the best tools available to address all the changes CRISPR makes that could be dangerous for patients.
The issue is that every time CRISPR edits a gene, it cuts a strand of DNA code that controls how the gene functions. The body responds by trying to repair the double-stranded break. It engages a biological repair process that reconnects those severed ends often unfaithfully creating random insertions and deletions (indels) of DNA code. The result is a new line of code that might have no impact whatsoever on how our body functions. But there is increasing evidence that sometimes it does have an impact. That new sequence enables a gene to start producing a completely novel protein—with unknown risks for patients.
To be clear, this is not the remote “off-target” risk of CRISPR straying far afield from its intended target to randomly alter some other part of the genome. This event can potentially happen whenever CRISPR lands right on its intended target.
CRISPR compute
The solution is simple. Anyone exploring CRISPR as a possible medical treatment should be working from the start to identify all the indels that occur around the edited target. Do they end up programming a gene to do something we don’t want it to do?
This kind of analysis appears to have been conducted for the Vertex sickle cell treatment. The problem, as I discovered several years ago, is that there is a disconcerting absence of affordable, effective tools for revealing the full range of indels generated by a CRISPR edit.
A few years ago, we were fortunate to have a young computer scientist, Rohan Kanchana, on our team. Kanchana produced an app that we now use to evaluate indels that we simply call DECODR (Fig. 1). The fact that we had to develop DECODR at all is a clue that this issue is a blind spot for the field. As we noted in a 2021 study published in The CRISPR Journal, DECODR helped us see that “the unpredictability and diversity” of gene mutations produced by indels should raise a caution flag for the race to develop CRISPR therapies.
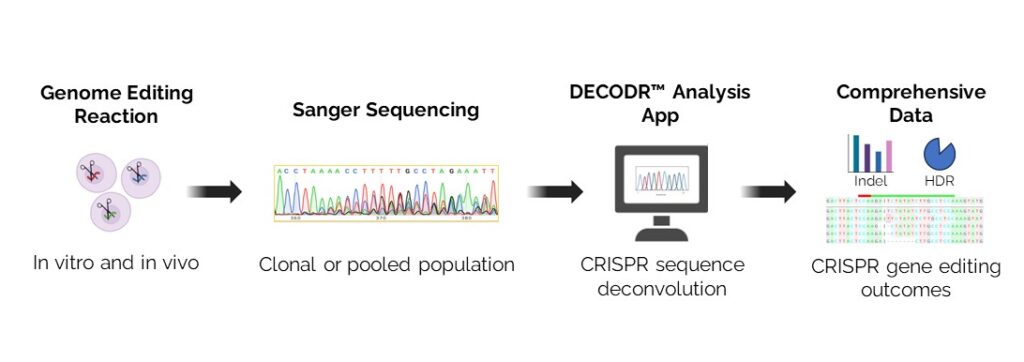
We have since made DECODR freely available to universities and research organizations. The frequency of downloads is an indication of greater attention to this area of CRISPR risks. I also was pleased to see that recent studies in Scientific Reports and Cells gave DECODR high marks in comparison with other software tools including TIDE, ICE, and Indigo. But I was surprised that the same study found CRISPR researchers often use tools that provide an incomplete picture of DNA changes that could end up affecting gene function, sometimes ported by the companies that do their analyses. We will continue to make DECODR available to the field and I encourage others to develop new precision tools to assess the genetic diversity of on-site indels.
The Vertex sickle cell breakthrough is an indication that we are on the threshold of a new era in which CRISPR allows us to treat or even cure a wide range of confounding conditions and diseases. But history says we need to do everything possible to game out all potential risks, which can vary widely from treatment to treatment and even patient to patient. That’s why now is the time for anyone involved in this field to embrace the best tools available for determining that a CRISPR edit will not harm the patients we are trying to help.
Eric B. Kmiec, PhD, is the founding Executive Director of the ChristianaCare Gene Editing Institute in Newark, Delaware. Email: [email protected]