If you describe the development of an antibody drug conjugate (ADC) in simple terms, you risk sounding as if you’re in an old Monty Python sketch, the one called “How to Do It,” which showed presenters offering ludicrously blithe guidance to those who would learn to play the flute, split the atom, or erect a box girder bridge. Saying that building an ADC involves combining three basic elements—an antibody, a linker, and a payload—is hardly less facile than saying that if you wish to play the flute, you should “blow in one end and move your fingers up and down the outside.”
The problem is that antibodies, linkers, and payloads all require different kinds of expertise. Worse, combining these elements is a specialty unto itself, as is establishing the biophysical properties and clinical effects of a finished ADC, which may in fact be a cluster of closely related ADCs, that is, ADCs with different drug antibody ratios (DARs). Yet another specialties involve ushering ADCs to clinical trials and bringing ADC production up to commercial scale.
For many developers of ADCs, the problem of marshalling all the necessary expertise is best left to contract manufacturing and development organizations (CDMOs). CDMOs are familiar with difficulties peculiar to developing and manufacturing ADCs, such as handling highly cytotoxic payloads, staging bioconjugation reactions with optimized antibodies, characterizing ADCs, integrating multiple workflows (or managing far-flung supply chains), coordinating with clinical investigations, and securing adequate manufacturing capacity.
CDMOs are also on the forefront of linker technology, which is crucial to the development of next-generation ADCs. Unlike first-generation ADCs, which rely on stochastic conjugation, next-generation ADCs implement site-specific conjugation. So, instead of attaching payloads to an antibody’s natural lysines or cysteines, and potentially generating a range of ADCs with different DARs, site-specific conjugation technology attaches payloads to selected sites, such as nonnatural amino acids, and generates homogenous constructs.
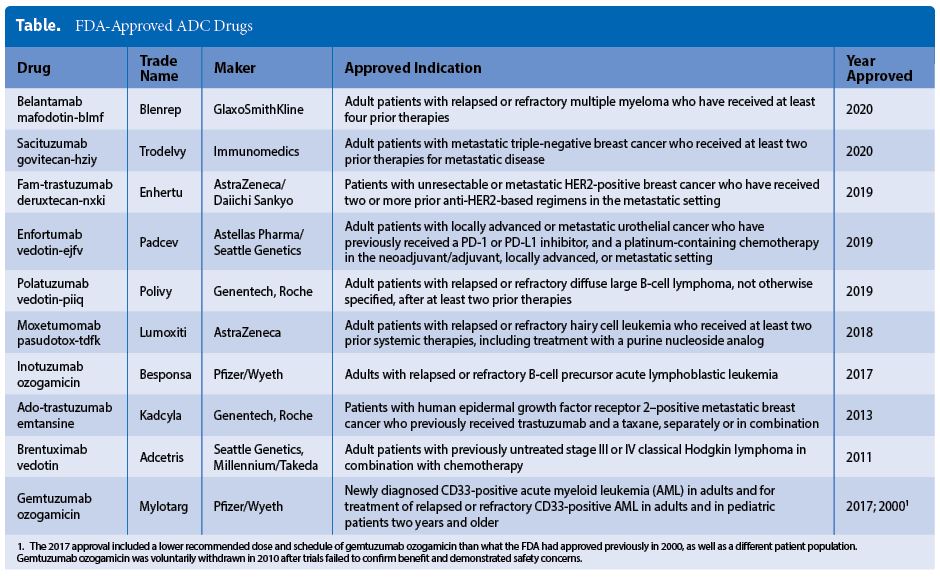
Next-generation ADCs have yet to join the list of FDA-approved ADCs—a list that currently includes just 10 drugs (Table). And relatively few of the 281 “antibody drug conjugate” studies listed on clinicaltrials.gov qualify as next-generation ADCs. Nonetheless, next-generation ADCs promise greater stability, safety, efficacy, and manufacturability, provided conjugation difficulties (such as multistep reaction sequences or long reaction times) and analytical challenges can be overcome.
Persistent challenges
Even if the difficulties specific to next-generation ADCs are set aside, other, more general difficulties remain. “ADCs come with a complex supply chain,” says Iwan Bertholjotti, director of commercial development, Bioconjugates, Lonza. “Different building blocks are required, including technologies for biologics and small-molecule drugs.” He adds that technological competencies to a high degree are required if cytotoxics are to be handled safely.
“Different scales, up to and including dedicated supply solutions, are typically required to manage the lifecycle actively and supply the clinic and the market as needed,” he notes. “Professional preparation of the registration process [prevents] surprises in the time-critical phase to move a new drug to market.”
Because meeting even general ADC challenges is difficult, “more than 70% of ADC projects are outsourced to CDMOs,” observes Matthias Bucerius, PhD, head of actives and formulation, MilliporeSigma. “Typically, 5 to 10 CDMOs across the globe are involved in the development and production of ADC programs.”

As a CDMO, MilliporeSigma maintains a comprehensive service portfolio. “It combines the crucial steps of the drug development and production: monoclonal antibody solutions, linker, payload, and the final conjugation—all from a single source,” asserts Bucerius. “[Our] experience and capabilities, which cover both clinical and commercial manufacturing, can help reduce risks and streamline processes, accelerating the work of getting therapies to patients.”
Last September, MilliporeSigma announced a $65 million expansion of its highly potent active pharmaceutical ingredient (HPAPI) and ADC manufacturing capabilities and capacity at its facility near Madison, WI. The expansion includes a new 70,000-square-foot commercial building that will be one of the largest dedicated HPAPI manufacturing facilities specifically designed to handle single-digit nanogram occupational exposure limit materials.

“Facility design,” notes Zhala Tawfiq, senior associate, process development and tech transfer, ADCs, Ajinomoto Bio-Pharma Services, “requires proper engineering controls to provide product and personnel protection from the highly potent compounds. This type of facility is a substantial capital investment, which is why most ADCs are manufactured at CDMOs rather than at in-house drug-developer facilities.
“Most smaller companies, and even some larger companies, do not have enough of a pipeline to justify the level of facility investment needed for ADC manufacturing and/or cannot keep the facility fully utilized to justify the asset. In addition, the supply chain for manufacturing ADCs is complex, including linker/toxin manufacture, antibody manufacture, conjugation/formulation/quality control and stability testing, and fill finish. The more of these supply-chain elements a CDMO can offer at a single site, the better for the client.”
Novel conjugation technologies
With next-generation ADCs, there is an opportunity to offer wider therapeutic windows. “We will see novel drugs that are based on new proteins partly engineered to allow site-selective conjugation, new hydrophilic linkers, and new payloads,” says Bertholjotti. “The field for complex proteins is wide, and we will see different modalities beyond ‘classical’ ADCs to address unmet medical needs.”
“By offering technology to provide inherent therapeutic window enhancement, wide drug linker applicability, native antibody compatibilities, and ease of manufacture at an early stage,” elaborates Tawfiq, “clients can directly benefit during downstream manufacturing from the familiarity and expertise of the CDMO to ease manufacturing. For instance, Aji Bio-Pharma offers AJICAP™, a direct chemical site-selective conjugation method of intact antibodies. With AJICAP, not only are antibody modifications well tolerated, but also compatibility with native antibodies is retained.
“This technology is flexible in that it accommodates even notoriously hydrophobic payloads, as well as both thiol- and azide-type conjugations to achieve DAR2 and DAR4 ADCs. Compared to stochastic conjugations, there are benefits via expansion of therapeutic windows with improvements in pharmacokinetics, safety, and efficacy. Finally, it is straightforward to manufacture, with short conjugation reaction times. All of this combines dramatic shortening of both early-stage timelines in ADC product candidate selection and downstream manufacturing of clinical materials if incorporated at early stages.”
The relative merits of the ever-growing list of site-specific conjugation approaches can be difficult to assess, suggests Penelope Drake, PhD, head of R&D, Bioconjugates, Catalent. “The ADC, she continues, “must be efficacious and well tolerated, features that depend on structural details such as conjugation site and linker stability. Important considerations that are often overlooked include process scalability and ADC manufacturability.
“Cost of goods can be a differentiator, as various site-specific conjugation approaches differ broadly in their requirements for GMP materials. Knowing how to evaluate the elements constituting an ADC program is not necessarily obvious, and guidance from CMDOs can go a long way toward helping customers quickly move projects forward.”
Catalent has demonstrated its technical expertise in the development of a clinical-stage ADC (TRPH-222, in a Phase I trial for relapsed/refractory B-cell lymphoma) and a pipeline of other ADC clinical candidates. Besides Catalent’s general ADC expertise, customers may access the CDMO’s proprietary SMARTag® technology. According to Drake, it encompasses site-specific conjugation, robust manufacturing, and highly stable linker chemistry that yields efficacious, tolerable drugs.
“We work with customer partners in the early R&D stage to set target candidate product profiles, to design and test ADC panels, and to select leads for further evaluation,” she asserts. “We help the customer understand what analytical assays are necessary and phase appropriate, design and execute those studies for them, and guide them through the data interpretation. At the same time, we support the CMC process, helping the customer drive toward a successful IND.”
What’s next?
Arguably, ADCs were conceived back in 1900, when Paul Ehrlich suggested that cytotoxic drugs could be developed that would target only tumor cells, sparing healthy cells. What we today recognize as ADCs appeared more recently. Twenty years ago, the ADC called gemtuzumab ozogamicin (Mylotarg) was approved by the FDA for acute myeloid leukemia. (Mylotarg was withdrawn in 2010 but brought back to the market in 2017 after changes to the drug’s dosing regimen.)
Today, just 10 ADCs have secured FDA approval. Yet ADC development is lively, with dozens of firms participating. “ADCs have experienced incredible growth over the last decade, and we expect this demand to continue,” says MilliporeSigma’s Bucerius. “The value of the global market for ADCs is expected to reach $15 billion by 2030. The FDA’s approvals in recent years also demonstrate their promise as a targeted therapy.
“We see the ADC market expanding significantly in the coming years both within oncology and beyond oncology. There are programs being evaluated for inflammatory, antimicrobial/antibacterial, and other indications as well. We’re also going to see a shift to less toxic and less potent payloads.”

Lonza’s Bertholjotti concurs: “Many novel bioconjugation drugs with a wide range of therapeutic targets beyond oncology are lined up in the preclinical and clinical stages and will provide solutions to address different unmet needs moving forward.”
Development experience and lessons drawn from clinical failures over the past 20-plus years, suggests Aji Bio-Pharma’s Tawfiq, are supporting “an expansion, perhaps a renaissance, in the bioconjugation and biological drug development industry. This trend is clearly evident in the examinations of clinical trial successes and in the new approaches entering late-stage preclinical and early-stage clinical development.”
“There has been growing consideration to the use of ADCs as therapeutics outside of the traditional oncology and hematology fields, for example, in autoimmune therapies,” she details. “An expansion of the traditional ADC field into other promising modalities that utilize the targeting nature of antibodies to deliver various payloads to the tumor microenvironment is an area of significant research and development efforts currently.”
Recent ADC approvals, adds Catalent’s Drake, have generated renewed interest and investment in the modality. “Accordingly, the science will continue to progress as we collectively learn more about how to make and dose these drugs,” she continues. “Structure-activity relationship mapping and improved linker design will lead to ADCs with ever-wider therapeutic windows, dramatically reducing side effects while maintaining efficacy. This in turn will lead to the expanded use of ADCs as part of combination therapy treatments, further improving patient outcomes and quality of life.”