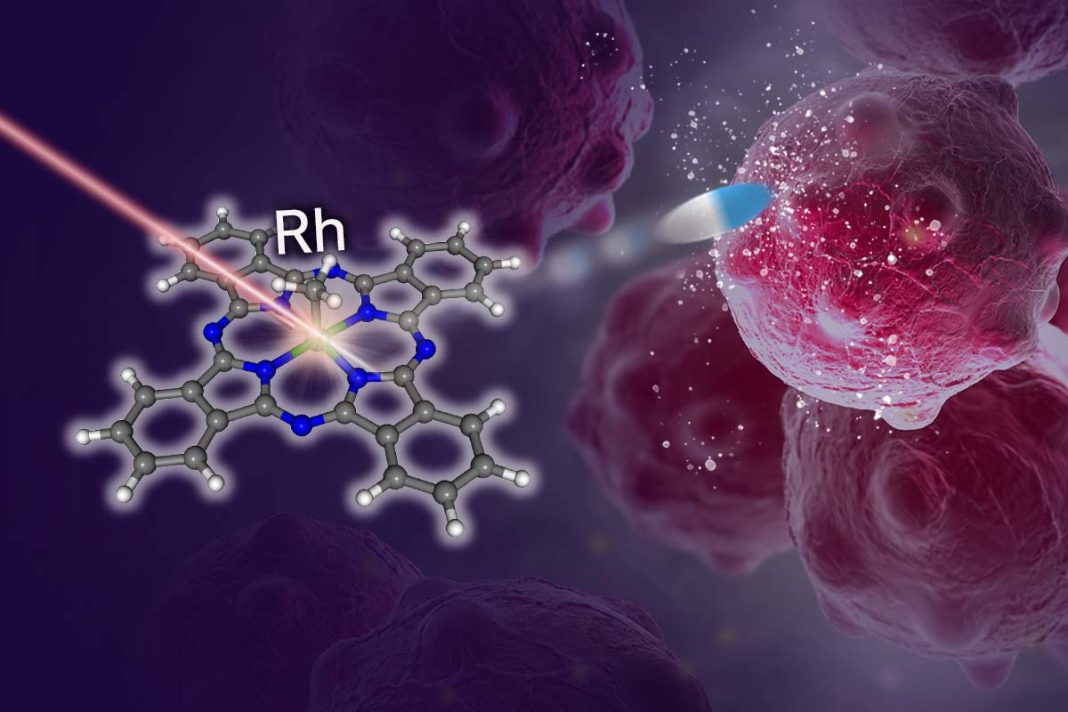
Researchers have developed a new photo-uncaging system for activating cancer-fighting agents at the site of the tumor. The system involves the use of organorhodium(III) phthalocyanine (Pc) complexes, which are activated by lasers but stable under ambient light. Importantly, unlike existing systems, they are effective in the low-oxygen environments typical of many tumors.
The work was carried out by a team from the Institute of Industrial Science at the University of Tokyo. They published their work in Chemical Communications in an article titled, “Two-Photon, Red Light Uncaging of Alkyl Radicals from Organorhodium(III) Phthalocyanine Complexes.”
One approach to treating cancer is photodynamic therapy, in which light is used to activate a cancer-fighting agent in situ. However, conventional photodynamic techniques depend on the formation of reactive oxygen species to destroy tumor cells, and many tumors contain environments that lack oxygen.
“To address these issues, the use of a photo-uncaging system has emerged as a promising alternative, in which the tumor-selective release of functional, long-lived active agents is expected to enhance the therapeutic effect of the treatment,” wrote the research team in their article.
The new system uncages alkyl radicals, which are capable of inducing cell death both with and without the presence of oxygen. Alkyl radicals are converted into terminal aldehydes in the presence of oxygen, and these terminal aldehydes can also induce cell death.
“[W]e describe a new platform based on organorhodium(III) phthalocyanine (Pc) complexes for the photochemical generation of an alkyl radical/aldehyde via a stepwise two-photon excitation under red light irradiation,” the team wrote. “These complexes were stable during synthetic, purification, and measurement processes but could be activated by a nanosecond pulsed laser.”
This platform represents an improvement on other photo-uncaging systems, opening exciting new avenues for the treatment of cancer.
“Our new technology could allow the photochemical generation of a wide variety of alkyl radicals and aldehydes, making possible the site-selective release of various bioactive molecules,” said Kazuyuki Ishii, D.Sc, professor, University of Tokyo and senior author on the paper.
Uncaging occurs by exposure to low-energy tissue-penetrative red light. “The organorhodium(III) phthalocyanine (Pc) complexes we developed are highly stable under ambient light during the processes of synthesis, purification, and measurement, but can be activated by a laser that gives out nanosecond pulses of red light,” explained lead author Kei Murata, PhD, research associate at the University of Tokyo.
The nanosecond-pulsing lasers (pulsing for a billionth of a second) are relatively easy for medical staff to handle.
During the course of the study, the researchers showed that the compounds that were released after the organorhodium(III) phthalocyanine (Pc) complexes were activated showed toxicity to HeLa cells, a cell line developed from cancer, indicating that these compounds would have the ability to fight cancer if released inside a tumor.