Researchers have found that the flexibility of the structure between the arms of an antibody—the so-called “hinge”—influences the strength of the immune response that it elicits. This finding could be used to design antibody drugs that are more effective in the fight against cancer.
The study, led by researchers from the University of Southampton, was published in a Science Immunology article entitled “Hinge disulfides in human IgG2 CD40 antibodies modulate receptor signaling by regulation of conformation and flexibility.”
Antibody drugs that stimulate anti-tumor immunity are transforming cancer treatment. But their development has been hampered by a lack of understanding of how to stimulate receptors at the right level.
In previous work, the Southampton team showed that the hinge structure of an antibody called IgG2 changed over time in a process termed “disulfide switching.” IgG2 binds to the receptor CD40, a potential target for cancer immunotherapy.
They also found that IgG2 was more active than other antibody types, making it uniquely suited as a template for pharmaceutical intervention. But they had not ascertained exactly why it was more active.
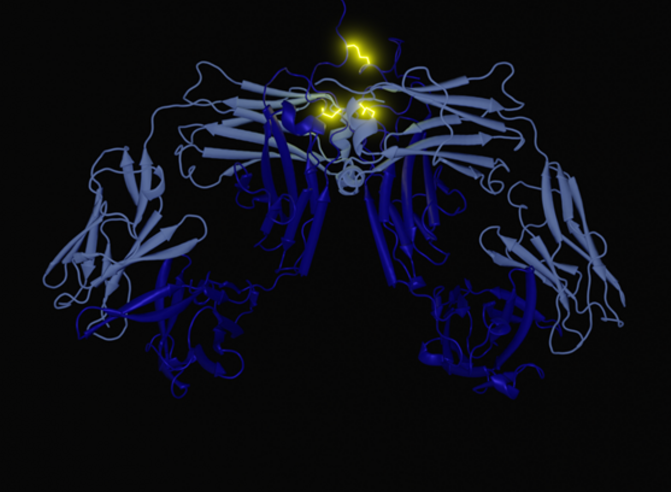
In their latest work, the researchers studied how disulfide switching alters antibody structure and activity. “Our approach was to analyze the structure of the antibody in atomic detail, using the method of X-ray crystallography,” explained Ivo Tews, PhD, associate professor of structural biology at the University of Southampton. “While the resulting picture was very accurate, the information on how they move their ‘arms’ was missing, and we needed an image of the antibody in solution, for which we used an X-ray scattering approach called SAXS. We then used mathematical models and a chemical-computing approach to analyze the data.”
Their work revealed that the activity of the antibodies varied, depending on the pattern of disulfide bonds in the IgG2 hinge region. The more compact, rigid antibodies, which featured a disulfide crossover, were more active than their flexible counterparts.
“The least-flexible variants adopt the fewest conformations and evoke the highest levels of receptor agonism,” explained the researchers in their article.
This agonistic (or stimulatory) activity may have significant biological and therapeutic implications. “We propose that more rigid antibodies enable the receptors to be bound closer together on the cell surface, promoting receptor clustering and stronger signaling for activity,” explains Mark Cragg, PhD, professor of experimental cancer research at the University of Southampton. “This means that by modifying the hinge, we can now generate more or less active antibodies in a more predictable way.”