Researchers at the University of Florida College of Medicine have uncovered how common age-related changes in the blood system can exacerbate certain colon cancers. Their findings also suggest how these effects may be therapeutically targeted to reduce tumor growth and improve patient survival.
Their new study is published in the Journal of Experimental Medicine in an article titled, “Hematopoietic-specific heterozygous loss of Dnmt3a exacerbates colitis-associated colon cancer.”
As we age, the hematopoietic stem cells that reside in the bone marrow and give rise to all of the body’s different blood cells gradually acquire mutations in their DNA. Most of these mutations have no effect, but some can enhance a particular stem cell’s ability to survive and proliferate, resulting in large numbers of blood cells that carry the same mutation, which is known as clonal hematopoiesis.
Clonal hematopoiesis is even more frequently seen in patients with many other types of cancer outside of the blood system and is associated with faster tumor progression and shorter survival times.
“However, whether the presence of clonal hematopoiesis causes the aggressive phenotype of unrelated solid tumors has not been vigorously addressed,” said Olga A. Guryanova, MD, PhD, now an associate professor at the University of Florida College of Medicine and a member of the University of Florida Health Cancer Center, who led the new JEM study.
“Clonal hematopoiesis (CH) is defined as clonal expansion of mutant hematopoietic stem cells absent diagnosis of a hematologic malignancy,” wrote the researchers. “Presence of CH in solid tumor patients, including colon cancer, correlates with shorter survival. We hypothesized that bone marrow–derived cells with heterozygous loss-of-function mutations of DNMT3A, the most common genetic alteration in CH, contribute to the pathogenesis of colon cancer. In a mouse model that combines colitis-associated colon cancer (CAC) with experimental CH driven by Dnmt3a+/Δ, we found higher tumor penetrance and increased tumor burden compared with controls.”
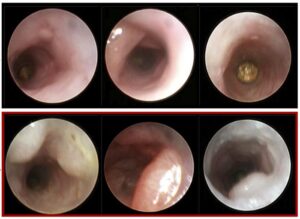
The team generated mice with clonal hematopoiesis by transplanting them with blood stem cells lacking one copy of Dnmt3a, the most frequently mutated gene in clonal hematopoiesis patients.
The team observed that CAC occurred more frequently, and developed more rapidly, in mice with clonal hematopoiesis, resulting in larger tumors with a worse histopathology.
The researchers determined that one way clonal hematopoiesis promotes the development of CAC is by increasing the number of blood vessels that supply the intestinal tumors with the nutrients and oxygen they need to grow.
Blocking the formation of these extra blood vessels with axitinib, a drug approved by the FDA to treat advanced kidney cancer, inhibited the growth of CAC tumors in mice with clonal hematopoiesis.
“Our results show that alterations in Dnmt3a in bone marrow stem cells can have profound impact on the development of CAC through multiple mechanisms, some of which may be therapeutically targetable,” Guryanova said. “Our findings, for the first time, solidify the causal relationship between clonal hematopoiesis and the severity of solid tumors and identify potential therapeutic strategies.”