Viral vectors are the essential drivers for gene transfer protocols. What’s more, the “library of viral vectors needs to be optimized based on their application for proper gene delivery to transfer gene sequences into human cells both ex-vivo and in-vivo,” says Rachel Legmann, PhD, director of gene therapy and viral vectors at Pall’s Gene Therapy and Viral Vectors Program.
“During optimization, the major focus is on improving patient safety by significantly reducing the vectors’ cytotoxicity and immune response,” she explains. “Other critical areas include enhancing the efficiency of cell transduction and building appropriate lengths of genetic material to accommodate various sizes of the target genes.”
This involves targeting the viral vector to specific cell types as well as boosting the longevity of transgene expression by adopting various promotors. The manufacturing process for production is developed specifically for the client in a small-scale bench top platform, and then scaled up, working with Process Development Services, to achieve appropriate scale.
Next, Legmann and her team transfer the developed manufacturing process of vector production and purification to a manufacturing site for generating adequate quantities of the drug for clinical trials, while retaining its critical properties.
“Like many others in the industry we are on a Bioprocessing 4.0 journey, assembling solutions and assessing the impact,” adds Loe Cameron, senior director of analytics & controls. “Our research is focused on how we can provide these tools in a way that does not negatively impact the performance of our technologies, while working closely with users of these systems to achieve that goal.”
Pall has installed automation systems in their process development labs which mimic many of the functions found in full scale manufacturing. This allows customers to more effectively predict the future of their process, says Cameron. The system is connected to a centralized historical database, allowing a rigorous exploration of how solutions such as soft sensors, modeling and advanced process controls can improve viral vector processes at each scale, and over the full life of the process.
“However,” Cameron continues, “It is important to remember that this has a cascading effect, since as we install more sensors to support the automation of our processes, vast quantities of data are generated, requiring that the analysis be automated as well. Recognizing that the use of these powerful technologies offers great potential, we strive for a balance of expanding to the right level of complexity, to clarify rather than complicate the system.”
Legmann went on to consider the power of their technology solutions for both adherent and suspension culture to overcome processing challenges in order to simplify the journey from laboratory to an industrial scale and from process development to regulated production.
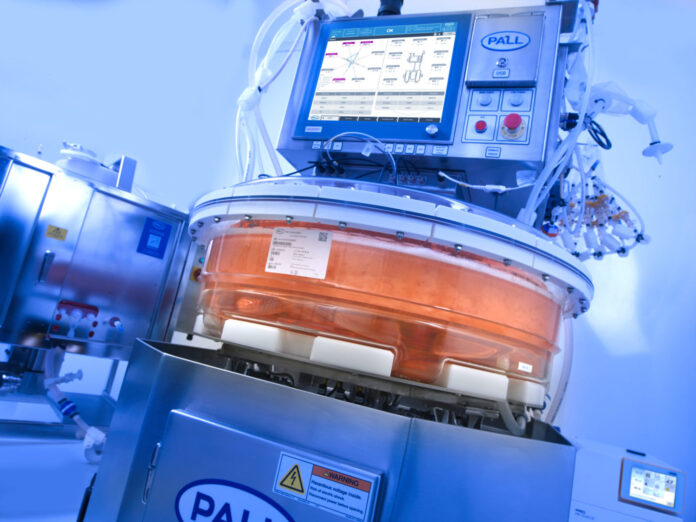
“The iCELLis® Nano bioreactor was designed to enable customers to complete process development and small-scale activities with assurance that these processes will perform just as well at commercial iCELLis 500 bioreactor scale,” points out Legmann. Both systems support viral vector production using adherent cells.
The iCELLis bioreactor as having a fixed bed design that enables various simplified manipulation steps during the culture such as simple media change and perfusion process steps, according to Legmann. Cell lysis, wash, and harvest in the fixed bed provide the downstream with a low turbidity crude product yield.
Legmann also mentioned the Allegro™ STR suspension bioreactor for viral vector production, available with for process development to commercial manufacturing scales (50 to 2,000 L). “This bioreactor portfolio offers low shear mixing support for HEK293 growth with minimum clumping and therefore better transient transfection efficiency,” she adds.