Artificial intelligence appears poised to transform many aspects of our lives, and drug discovery is no exception. By removing much of the trial and error from drug discovery, AI reveals new targets and tactics for treating diseases.

X-Chem
“AI is an umbrella for different types of machine learning models that can be trained toward different goals,” says Noor Shaker, PhD, senior vice president and general manager at X-Chem. For example, AI encompasses models for supervised and unsupervised learning.
Supervised learning algorithms are trained on examples. “This requires an existing body of large, labeled—annotated—datasets that can be used to train a machine learning model that can be later used to assign labels for new data points,” Shaker notes. “Such systems are used in prediction tasks, such as the prediction of drug-target interactions or drug profiles.”
Unsupervised learning algorithms, in contrast, explore data for new patterns with no expectations. These algorithms, Shaker points out, “are used to learn from what we know about a specific domain, with the aim of creating and expanding the domain space.”
The AI family of technologies offers many potential benefits in drug discovery. “AI can be used to design novel starting points for drugs by learning and generalizing from existing and human-designed drugs, and by exploring chemical spaces massively larger than those that can be explored by human or standard computational methods,” Shaker asserts. Equally important, AI can, she adds, be used in “optimizing those drug starting points to maximize the likelihood of their success—of their becoming medicines.”
A bundle of benefits
In general, AI makes drug discovery faster, more efficient, and less costly. “This is driven by the use of AI to reduce [the number of] laboratory experiments by predicting what might work and how to make these drugs,” Shaker says.
Other AI-based benefits could be even more useful. “What I find really exciting about AI in drug discovery,” Shaker emphasizes, “is the fact that we can now address diseases and targets that are known to be hard to drug. [AI can do that] by expanding our cognitive abilities and proposing chemical starting points and modifications that may have eluded human chemists.”
One way that scientists at X-Chem use AI involves the use of DNA-encoded library technology. This technology “generates massive libraries of billions of compounds and [determines] whether they [contain compounds that] interact with certain biological targets,” Shaker relates. “This information is a goldmine for supervised AI systems that can learn drug-target associations and can then be used to identify compounds that are fast and relatively cheap to make and yet have a high probability of success.” X-Chem has been using this approach on projects for various clients. According to Shaker, this approach increases the speed and reduces the cost of drug discovery.
Increasing use
Grant Wishart, PhD, senior director, small molecule drug discovery and Logica lead, Charles River Laboratories, says that the use of AI and machine learning (ML) technology is instrumental in the company’s overall digital journey. More specifically, AI/ML is helping Charles River and its clients leverage domain expertise across many data-rich areas in drug discovery such as digital pathology, hit finding, and lead optimization.

Charles River Laboratories
The type of AI that is used depends on the task. For early-stage, small-molecule drug discovery, Charles River uses generative and predictive technologies. “Generative models are being used to design chemical structures that are optimized for specific properties, whereas predictive models are being built for parameters of interest such as on-target potency, early absorption, distribution, metabolism, excretion, and selectivity,” Wishart explains.
Charles River develops some methods in-house, but it also has partnerships with Valo Health and Valence Discovery—companies that Wishart describes as “leaders in the AI/ML drug discovery space.” With Valo Health, for example, Charles River created Logica, which provides hit finding through candidate selection in small-molecule drug discovery.
In a recent internal pilot study, the Logica team aimed to identify and optimize inhibitors of a specific tyrosine kinase protein, Wishart says. The study used a combination of high-throughput screening, DNA-encoded library screening, and AI/ML-driven hit finding to reveal multiple interesting chemotypes. The study also used AI/ML-guided chemistry optimization cycles to prioritize these chemotypes for further optimization.
From fake to functional
Another company using AI is Insilico Medicine. Indeed, the company has been using generative AI since 2014. “We originally specialized in generative biology—generating ‘fake’ biological data using generative models,” recalls Alex Zhavoronkov, PhD, the company’s founder and CEO. This work, he continues, generated “billions of biological profiles based on transcriptomics, methylation, and other omics data types with the desired properties”—information that can be used in the company’s AI platform.

Insilico Medicine
In 2016, Insilico Medicine expanded into generative chemistry. A couple of years later, the company started combining biology and chemistry to build more complex models. To accomplish this work, Insilico Medicine used recurrent neural networks and custom tools such as the company’s patented mutual information autoencoder.
Other AI-based software tools developed by the company include PandaOmics, Chemistry42, and InClinico. They can be used for target discovery, drug design, and clinical trial prediction, respectively. “These tools work together in an end-to-end feedback loop and together make up our AI drug discovery platform, Pharma.AI,” Zhavoronkov says. “Recently, we opened a fully autonomous AI-run robotics laboratory that allows us to further accelerate our research and validate our software.”
Working in partnership with AI experts from the University of Toronto’s Acceleration Consortium, Insilico Medicine used these tools to generate a novel inhibitor for CDK20, which could be a drug target for hepatocellular carcinoma. According to Zhavoronkov, the partners evaluated nearly 9,000 designed molecules based on 54 unique scaffolds to find “two compounds that displayed strong potency for the intended target.”
Searching in single cells
Cellarity is demonstrating that in drug discovery, AI models aren’t limited to the exploration of molecular interactions. “Our AI models utilize data at single-cell resolution to identify cell-state transitions that drive disease, and to identify compounds that can revert these disease-state transitions,” says Fabrice Chouraqui, PharmD, CEO of Cellarity and CEO-partner of Flagship Pioneering. “This uncovers novel biology, even in diseases with no known targets.”
Rather than focus on a single molecular target, Chouraqui emphasizes, AI investigations can encompass the cell, enabling “a much fuller representation of disease.”
Cellarity uses its AI systems to advance small-molecule drug discovery. For sickle-cell disease, for example, Cellarity “generated a single-cell map of hematopoiesis and identified cell behaviors that are tied to the production of a protective form of hemoglobin,” Chouraqui reports. “We then engineered small molecules that directly induce these cell behaviors.”
Chouraqui notes that the resulting molecules exceed the impact of the existing standard of care and are “equivalent to gene therapy in vitro.”
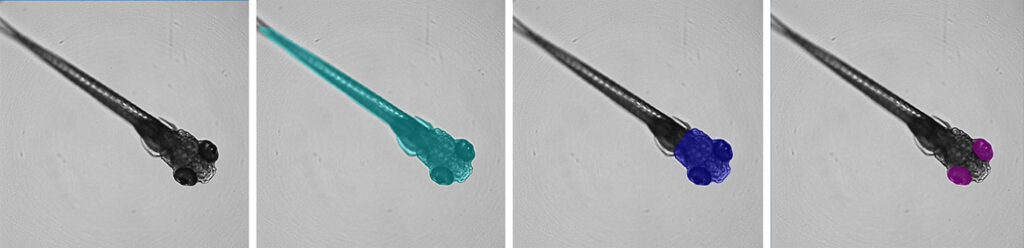
AI-automated imaging
Although microscopy serves as a powerful tool in drug discovery, including in identifying druggable targets and how potential drugs bind to them, its value depends on quantification. “You can capture images of cells that look very pretty, but as scientists, you want to describe the images in a quantitative manner,” says Angeline Lim, PhD, a senior applications scientist at Molecular Devices. “In response to perturbation, scientists might ask if a structure has changed in size, shape, or texture—all the visual information that can be represented numerically.”

Molecular Devices
In the past, scientists developed image analysis protocols to measure structures of interest. “It can all work great on one image but not on another image within the same experiment, and you have to go back to the drawing board and redo some of the numbers you’ve put in as parameters,” Lim explains. “When I first started using one of our AI tools, it felt so good.”
The company’s AI-based software provides Photoshop-like tools for image annotation. Essentially, the tools enable users to highlight objects of interest for analysis. “Give the software annotated images, let it learn from those images, and then apply that to the other images,” Lim continues.
When carefully trained, an AI-based software model can reveal changes that scientists might miss. These include cellular phenotype changes that occur in response to the environment or exposure to some perturbations, but that are too small for the human eye to discern.
AI-based image analysis can also find objects that scientists might overlook. Once, Lim trained her software to analyze organoids, which are 3D clusters of cells that resemble human tissue. Any bubbles in the microwell can obscure the organoids, so Lim trained the software on clearly visible objects. “When I ran the analysis and looked at the data, I was shocked,” she recalls. “I found organoids under the bubbles and at the well edges, which are typically dimmer. I changed the brightness, and I saw that something was there.”
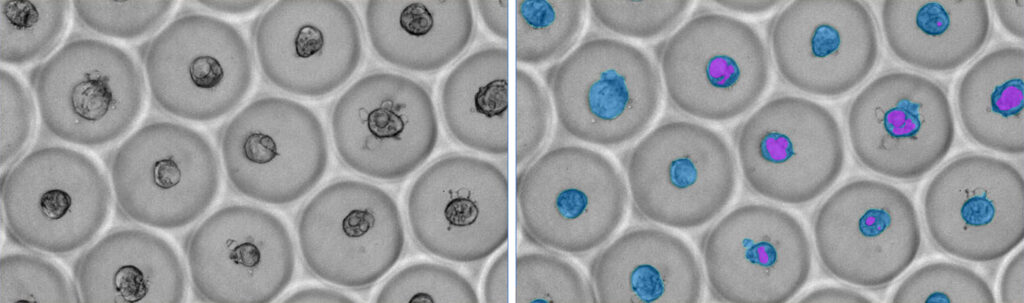
Finding new patterns, even unseen objects, is just the start of what AI can do for drug discovery. “Looking holistically, we know that drug discovery is hugely challenging, with high costs, long timelines, and high failure rates,” Wishart says. “Therefore, any technology that can increase the probability of success and reduce timelines is of immense interest.”



