Sponsored content brought to you by
In biology, there are multiple levels of structure, from whole tissues to single cells to subcellular. Interrogating each level requires increasing the power of resolution.
What Is Spatial Biology?
The rapidly evolving field of spatial biology investigates the spatial location and organization of gene expression in situ within each cell and structure of a given tissue sample. Maintaining the spatial context of biological data is crucial for understanding how cells organize and interact with their surrounding environment to drive various biological functions. Thus, spatial transcriptomics and spatial proteomics can be used to study healthy and diseased tissues by understanding the mechanisms behind the development, tissue heterogeneity, and therapeutic response.
Spatial Transcriptomics Technologies
Biology is inherently spatial—cell fate decisions depend on spatial relationships between cells, the interaction of immune cells within a tumor—and cannot be resolved optimally by bulk or single-cell analysis only. Several new technologies have been developed that aim to combine gene expression data with spatial context. Most of the spatial transcriptomics technologies available today have been built on in situ and scRNA-seq and can be primarily categorized into (1) NGS-based approaches that encode positional information onto transcripts before sequencing and (2) imaging approaches based either on in situ sequencing (ISS) where transcripts are amplified and sequenced in the tissue or fluorescence in situ hybridization (FISH) where imaging probes are sequentially hybridized in the tissue.1 This classification is not clear-cut, as some technologies may incorporate elements of both categories.
Spatial Transcriptomics Technologies: NGS-Based or Imaging-Based?
While both NGS- and imaging-based technologies are extremely powerful, each has its limitations. No single method can currently address all the desired parameters, such as high-throughput, sensitivity, resolution, and sample compatibility. High-throughput NGS-based methods are unbiased, as they capture all polyadenylated (polyA) transcripts, making them better suited for true discovery applications or scanning an entire tissue sample. However, with these methods that rely on a polyA pulldown, there is always the risk of missing lower abundance transcripts without compensation with respect to sequencing depth. By contrast, targeted NGS-based methods like Digital Spatial Profiling (DSP) used by the GeoMx® DSP System allow researchers to profile up to 18,000+ protein-coding genes—the whole transcriptome—while maintaining a wide dynamic range for detection of low- to high-expressing genes. GeoMx DSP works well on notoriously difficult Formalin-Fixed Paraffin-Embedded (FFPE) tissue sections and to date has been a valuable tool for research in oncology, COVID-19, developmental biology, and neuroscience. FFPE tissue sections that have been collected over decades and remain untouched sitting in biobanks can now be used for retrospective transcriptome analyses involving not only biomarker discovery but also atlasing of the whole transcriptome in different tissue substructures.
Why Not Both?
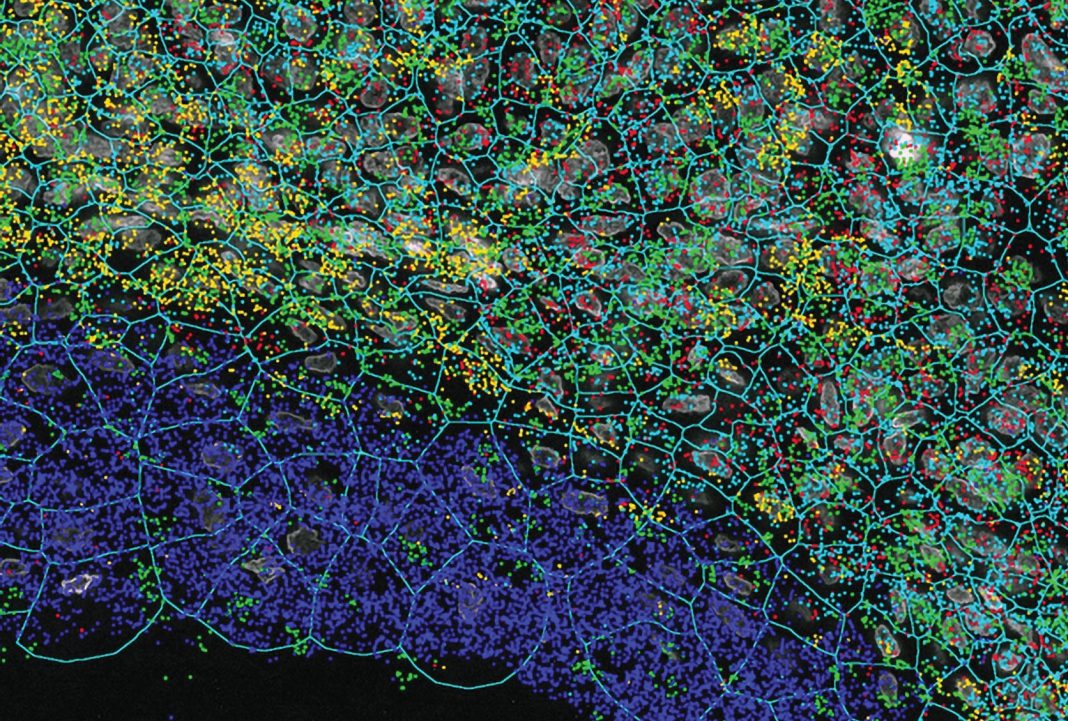
One of the emerging targeted and imaging-based technologies that bridge the gap between high plex, high throughput, and high resolution is NanoString’s CosMx™ Spatial Molecular Imager (SMI). CosMx SMI is a multiplex in situ-based system that provides single-cell and sub-cellular resolution on FFPE and fresh frozen tissue sections with quantification of up to 1,000 RNAs or up to 100 proteins. Because CosMx utilizes smart cyclic in situ hybridization chemistry with an “on” and “off” signal barcode design that overcomes molecular crowding, only a subset of RNAs or proteins is “on” in any given cycle, leaving room for continued expansion of more plex. With a tunable workflow from high-plex to high-throughput, CosMx enables not only the highest plex and unbiased whole-slide analysis, but also low-plex, high-throughput biology-driven analysis. The applications of CosMx are vast and versatile: it could be utilized to discover and map cell types, generate cell atlases, analyze cell-cell interactions, and discover single-cell biomarkers.
The CosMx SMI system is ideal for deeper single-cell exploration downstream of whole transcriptome analysis carried out on GeoMx DSP. These two systems can essentially be used for complementary experiments on a set of samples. As single-cell resolution spatial transcriptomics and proteomics pioneer the next frontier in spatial biology, our understanding of fundamental processes in biology will deepen and perhaps pave the way for next-generation diagnostic and pathology tools.2
References
- Rao A, Barkley D, França GS, Yanai I. Exploring tissue architecture using spatial transcriptomics. Nature 2021; 596(7871): 211–220.
- Aldridge S, Teichmann SA. Single cell transcriptomics comes of age. Nat. Commun. 2020; 11(1): 4307. Published 2020 Aug 27. DOI: 10.1038/s41467-020-18158-5.
Learn more about NanoString’s CosMx Spatial Molecular Imager and its capabilities