March 1, 2017 (Vol. 37, No. 5)
Fail Fast, Fail Cheap
Developing a new therapeutic molecule is a complex and expensive process with a high risk of failure at each step. Research suggests that aggregate costs per commercialized drug (including failures) are approximately $2.6 billion, with individual clinical trials costing up to $195 million.1 Failure represents an enormous financial risk.
Only 1 in 10 Phase I drug candidates (1 in 20 for oncology applications)2 makes it through the clinical trial process to commercial success. Decreasing costs associated with running clinical trials is a top priority for pharmaceutical companies to create sustainable drug development programs.
Biomanufacturing is one area where pharmaceutical companies can strive to reduce costs while maintaining the highest level of quality. Current biomanufacturing platforms typically rely on relatively inflexible, large-scale batch/fed-batch stirred-tank bioreactors. While these platforms are ideal for manufacturing hundreds of kilograms per year, they are not cost-effective for the sub-kilogram per year quantities needed for toxicology, animal studies, ex vivo, and early phase clinical trial-scale production.
Cell Culture Company (C3) has developed the AcuSyst® perfusion bioreactor systems, which allow for scalable, cost-effective production throughout the clinical trial process and beyond. The single-use systems reduce operational costs, decrease development timelines, and produce biopharmaceuticals of the highest quality.
Reduce Costs
C3’s perfusion bioreactors significantly decrease biomanufacturing costs compared to other commercial platforms by reducing capital expenditures, facility requirements, disposable costs, labor, raw materials, and process steps. By consolidating biomanufacturing process steps, C3’s AcuSyst bioreactors reduce costs in labor and materials. One key area of cost reduction is the seed train, where the AcuSyst systems require only a fraction of the culture steps compared to a conventional tank-based process. By eliminating seed-train steps, this approach saves both time and money.
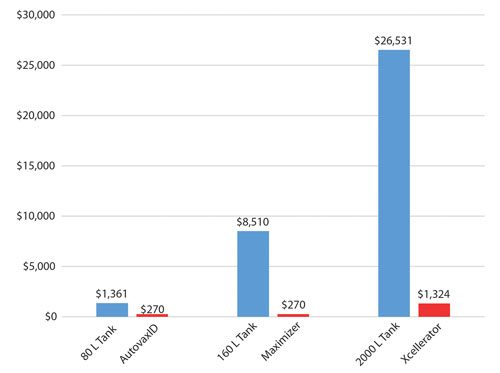
Figure 1 shows a comparison for estimated seed-train costs for equivalent stirred-tank runs compared to an equivalent AcuSyst perfusion bioreactor run. The seed train for fed-batch processes is typically carried out by first growing the cells in t-flasks, then scaling up through small tanks until a large enough volume has been reached for the final production scale.
Thus, several stages of cell growth must be accomplished before a tank run can begin, with higher costs and longer culture durations associated with higher target volumes. Skipping some of these steps with the AcuSyst perfusion bioreactors reduces time, labor, and materials on every production run.
C3 utilizes three linearly scalable AcuSyst bioreactors, each containing cartridges run in parallel to increase production. These include the AutovaxID™ (1 cartridge), the Maximizer™ (2 cartridges), and the Xcellerator® (6–20 cartridges). The AcuSyst hollow fiber cartridges are typically seeded at a low density of 1 × 106 cells/mL in about 0.5–1 L of media per bioreactor cartridge. The AutovaxID is seeded with ~1 L of cells; the Maximizer requires ~2 L; and the Xcellerator ~10 L.
Data from previous manufacturing runs have indicated that the protein yield for a hybridoma cell line using the AutovaxID is the equivalent of an 80 L tank. The two-cartridge Maximizer is thus the equivalent of a 160 L tank, and the Xcellerator is the equivalent of a 1,600–2,000 L tank. Figure 1 clearly shows the cost for the seed train of a tank system is significantly higher than that of the comparable AcuSyst systems.
C3’s perfusion bioreactors additionally save facilities and labor costs with a small footprint as well as automated monitoring and control systems, reducing direct hands-on labor time during bioreactor operation. AcuSyst bioreactors also require less media than other perfusion systems, thus saving on material costs. The reduced costs across both initial start-up and operations provides a strong competitive advantage for biomanufacturing with C3’s perfusion bioreactors.

Figure 1. AcuSyst perfusion bioreactors reduce seed-train costs. Estimated seed-train labor and material costs for stirred-tank and AcuSyst perfusion bioreactors assume a single production run, a media cost of $10/L for bulk-discounted serum-free media, $1/mL of supplements, and 30 min per day of labor for both tanks and AcuSyst bioreactors. For stirred-tank systems, estimated scale-up is assumed to be 50% of the final working volume.
Protect Quality
Maintaining consistency and quality of the final biopharmaceutical product is crucial. C3’s perfusion bioreactor systems provide cost-savings while maintaining the same level of product quality that is achieved using other production platforms.
AcuSyst technology, which includes automated monitoring, limits manual handling and maintains healthy, productive cells, which prolongs productivity nearly six times longer than fed batch systems. This is especially important for low-yielding or more challenging cell lines, which often benefit from longer, cleaner culture runs. In contrast, fed-batch tank systems provide a production environment in which waste is never removed and cell health cannot be maintained long term.
AcuSyst single-use bioreactor cartridges contain thousands of hollow fibers for culturing and growing mammalian cells to very high densities. The cellulose acetate fibers create two compartments: the intracapillary space (IC) and extracapillary space (EC). Media flows through the IC space at a high rate, and flow between the IC space and the EC space occurs through pores in the fibers.
Cells are grown in the EC space where they are protected from shear forces generated by fluid flow. The ~60 kDa molecular weight cutoff of the hollow fiber pores prevents cell and product loss while allowing the exchange of essential nutrients and removal of waste. Product is continuously harvested from the EC space to allow for regular quality monitoring. In addition, a 0.2 µm pore in-line filter ensures no additional clarification is necessary, reducing time and costs associated with downstream steps.
Perfusion systems are being widely implemented to grow healthier cells that produce protein for longer periods to maximize productivity. Typical AcuSyst cultures maintain cell health and productivity between 60–120 days, whereas cell health in fed-batch tank run declines around 14–21 days.3
Throughout the duration of an AcuSyst culture run, parameters such as pH remain stable due to an automated monitoring and control system (Figure 2A). Stable supply of nutrients and removal of waste also promotes cell health, as demonstrated by stable maintenance of glucose and lactate levels (Figure 2B), and long-term continuous production of the protein of interest (Figure 2C). These consistent metabolic parameters support other findings4,5 that perfusion systems are better able to maintain cell health than batch or fed-batch systems.

Figure 2. Consistent metabolic parameters indicate long-term cell health and antibody production in AcuSyst bioreactors. (A) Two different cell lines in AcuSyst bioreactor cultures (1 and 2) maintain stable pH conditions throughout the run. (B) Glucose and lactate values from the same culture runs as in (A) grown in AcuSyst perfusion bioreactors. Levels remain constant throughout the 85-day (cell line 1) and 69-day (cell line 2) cultures. (C) Continuous IgG production over 15 weeks for two cell lines growing in AcuSyst Bioreactors. Not the same cultures as in (A) and (B).
Move Faster
C3’s AcuSyst bioreactors save time leading up to, during, and between manufacturing runs. As stated above, there are significant benefits associated with eliminating seed-train steps. Additionally, tank systems require time-consuming concentration of the final product in their downstream processing. This step is essentially eliminated when using AcuSyst bioreactors, which concurrently concentrate the product 100–200× during the harvest.
Finally, the AcuSyst bioreactors save time between clinical trial phases. Between phases, required scale-up of biomanufacturing can be a lengthy and expensive process. Traditional stirred-tank bioreactors are not linearly scalable and require re-validation as well as extensive optimization when moving to larger tank systems. When the AcuSyst platform is used, production is validated to a single cartridge. The platform is thus linearly scalable by adding bioreactor cartridges to run in parallel for larger production scales (Figure 3A).
In Figure 3B, two murine hybridoma cell lines producing monoclonal antibodies were cultured in all three sizes of AcuSyst bioreactors. Total yield of IgG per day was normalized to the number of cartridges in the bioreactor to determine production variability when scaling-up total culture volume. The Maximizer and Xcellerator each run multiple hollow fiber cartridges in parallel, and each cartridge produces approximately the same amount of IgG product as the single AcuSyst cartridge. Thus, the time, cost, and risk associated with scaling up manufacturing between clinical trial stages is greatly reduced.

Figure 3. AcuSyst Bioreactors are made linearly scalable by adding cartridges of the same size in parallel. (A) There are three AcuSyst bioreactors utilized according to scale needs: AutovaxID, Maximizer, and Xcellerator. (B) IgG yield from two hybridoma cell lines grown in three different AcuSyst bioreactors was normalized to mg of IgG produced per day in each cartridge. Production of IgG is consistent, even across systems where multiple culture cartridges are run in parallel to scale up total production.
Emily Wozniak, Ph.D. ([email protected]), and Kyle Biesecker, Ph.D., are project managers at C3.
References
1. DiMasi, Grabowski, Hansen. 2016. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 47: 20–33.
2. Clinical Development Success Rates 2006–2015. June 2016. BIO.
4. Cunha, Aguir, Silva, Silva, Sousa, Pineda, Peixoto, Carraondo, Serra, Alves. 2015. Exploring continuous and integrated strategies for the up- and downstream processing of human mesenchymal stem cells. J. Biotechnol. 213(10): 97–108.
5. Liu, Kimura, Jayapal, Wu, Trautwein, Mayer-Bartschmid, Jockwer, Barrett. 2017. Fed-batch culture process development: Implementing a novel nutrient additive for a robust, high-titer, scalable process. BioProcess Int. 12(8).
6. Pollock, Ho, Farid. 2013. Fed-batch and perfusion culture processes: economic, environmental, and operational feasibility under uncertainty. Biotechnol. Bioeng. 110(1): 206–219.