December 1, 2012 (Vol. 32, No. 21)
Combining Luminescence and Fluorescence Genetic Reporters to Assess Drug Effects
Cell-based assays are highly versatile tools for assessing cell response of biological and chemical stimuli and have great potential in the research lab. They can be tailored to evaluate many cellular and biochemical functions. They are used to discover and develop novel drugs and test the toxicity of new and old chemicals.
To further improve the biology of cells grown in vitro, cell technologies that allow a 3D structure toward a more organotypic tissue model are now being used more intensively. These culture systems are becoming increasingly complex as they are composed not only of a single-cell type, but incorporate several cell populations to mimic native tissue as closely as possible.
The cellular self-assembly approach for microtissues has been shown to be one of the most versatile technologies to generate a vast variety of different tissue types derived from either cell lines, primary cells, stem cells, or induced pluripotent stem cells. The basic concept of generating microtissues by cellular re-aggregation is to prevent adhesion to the culture dish, thereby allowing direct cell-cell interactions. The cells excrete endogenous cell-specific extracellular matrix, leading to the formation of spherical microtissues from cell lines, primary cells, and stem cells.
Multicellular tumor spheroids have been used for a long time in drug development as they display similar cell heterogeneity as in native tumors, such as oxygen profiles, nutrient gradients, metabolic activity, and proliferation. Although the scientific community widely appreciates the improved organotypic phenotype of tumor spheroids, their implementation in high-throughput screening assays has been hampered due to a lack of appropriate technologies.
HTS-Compatible Assays to Assess Drug Effects in 3D
Most biochemical assays were developed for use on monolayer models and need modifications depending on the tissue type due to high secretion of extracellular matrix. Moreover, when using complex models at an early stage, the costs and read-out speed have to be taken into account.
Lytic assays, such as the CellTiter Glo (Promega), have shown compatibility with the microtissue format to assess tissue viability. However, to get the most out of a microtissue-based assay, additional nonlytic read-outs can be added to discriminate effects on distinct cell populations in the model. To this end, InSphero has developed a specific assay plate to culture microtissues at a defined position.
The plate is compatible with the Tecan Infinite M200 PRO, which captures the signal of a whole well without the need for multispot measurements. Together with the use of highly sensitive reporter systems, this system has become an efficient and automation-compatible tool for assessing drug effects in microtissue models.
The Infinite M200 PRO is a versatile multimode reader, based on a sophisticated double-monochromator optic; it offers high sensitivity for all fluorescence- and luminescence-based measurements. In a previous study, the optical properties of the system were shown to perfectly match the necessary requirements for sensitive detection of fluorescent signals originating from 3D microtissues.
In addition to the unique design of its fluorescence optics, the Infinite M200 PRO is equipped with a separate and independent luminescence optic, developed to detect luminescent signal with the highest possible sensitivity, which allows convenient analysis of luminescence-based reporter signals, like those of the NanoLuc luciferase.
The smart design of Tecan’s i-control™ software enables multiplex measurements like those used in this study. Tecan’s robotic workstation, the Freedom EVO®, is capable of handling 3D microtissues and the Infinite M200 PRO multimode reader can be integrated into the system to optimize the process for high-throughput analysis.

The Infinite M200 PRO is a versatile multimode reader.
Discriminating Drug Effects in Multicell Type Models
In order to effectively discriminate the effects in multicell type models, either cell type-specific biomarkers must be used or the cells need to be engineered to express distinct reporter genes. Using cell populations with integrated reporter functionality allows the differentiation of effects on individual cell types and additionally reduces assay efforts.
Luciferase and fluorescent reporters are known to be useful tools for drug discovery and development. From the perspective of signal detection, the combination of luminescent and fluorescent signals in a multiplexing approach is preferable to a fluorescence-only multiplexing system. This is simply due to the fact that no cross excitation of various fluorescent dyes is possible, which significantly increases signal specificity.
Therefore, single- and dual-cell type microtissue models harboring a novel small, secreted luciferase, in this case NanoLuc luciferase, or a red fluorescent protein were evaluated. Far red fluorophores were preferred as the emitting light penetrates tissues better than chromophores with shorter excitation wavelengths (e.g., green fluorophores). This can be done either with fluorescent labels or with a combination of fluorescent and luminescent reporters to completely de-couple the signals of the two cell populations.
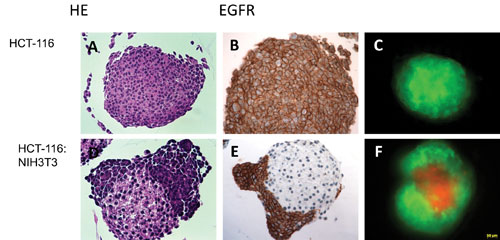
Figure 1. Immunohistology characterization of single-cell type colon cancer (A-C) and dual-cell type models (D-F) derived from a colon cancer cell line (HCT-116) and mouse embryonic fibroblasts (NIH3T3): The cancer cell population is characterized by positive epithelial receptor staining (EGFR) surrounding the connective tissue core (E). Expression of fluorescence reporters enables the discrimination of the two populations, HCT-116 expressing a green fluorescent (C) and the fibroblasts a red fluorescent protein (F).
Colon Cancer
In this study we present a novel approach to distinguishing drug effects in a co-culture model of colon cancer cells harboring the secreted NanoLuc luciferase (HCT-116-NanoLuc) and mouse fibroblasts harboring the red fluorescence protein (NIH3T3-RFP; cell lines were generated by Sirion Biotech).
The cells self-assembled to microtissues whereas the HCT-116 were enveloping the connective tissue core (Figure 1). Both reporters allowed for monitoring growth over time by either measuring the activity of the secreted luciferase or fluorescence intensity of RFP. Due to the high sensitivity of the NanoLuc system, small volume sampling (5 µL) was sufficient to assess the growth profile.
Pure NIH3T3 microtissues did not grow significantly over time, which is reflected by the stable fluorescence signal over time. Both endpoints were applied to assess the growth profile of the individual cell populations in the co-culture microtissue model. Whereas the luciferase activity was increasing, the fluorescence signal remained stable up to four days before slightly decreasing, which is a result of the increased thickness of the HCT-116 layers.
Together with assessing total ATP content, both endpoints were used for dose-response testing of three reference compounds: (i) Staurosporin, (ii) Cisplatin, and (iii) Taxol. In all three cases the compounds affected the cancer cells but did not show a severe impact on the stroma (Figure 2).
The combination of Tecan’s Infinite M200 PRO multimode microplate reader with InSphero’s organotypic microtissue tumor model gives a scalable read-out to assess drug sensitivity in complex multicell type 3D cell culture models, allowing long-term tissue-based analysis, for example, for cell-proliferation studies, and offers reproducible measurements over time.
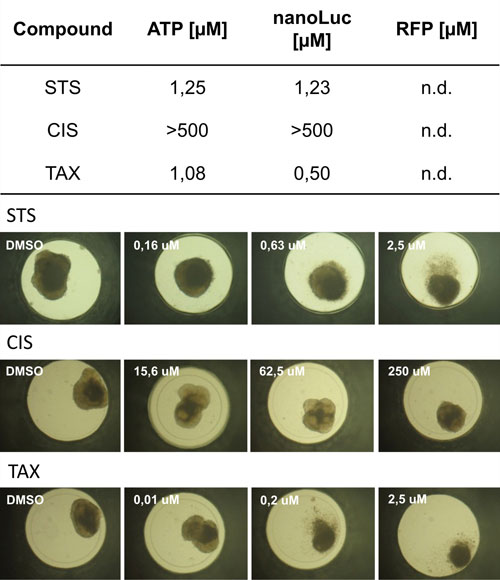
Figure 2. IC50 values calculated from dose-response curves applying Staurosprin (STS), Cisplatin (CIS), and Taxol (TAX) after 72h incubation time applying an endpoint combination of total ATP content, NanoLuc secretion, and fluorescent intensity measured with the Infinite M200 PRO multimode microplate reader. Whole microtissue IC50 values were calculated measuring intra-microtissue ATP content. IC50 values calculated from either established ATP measurements or secreted luciferase correlate very well. None of the compounds impacted the fibroblasts to calculate IC50 values. To further exemplify the impact of the compounds, bright field images display morphological changes with increased compound concentration.
C. Oberdanner ([email protected]) is application scientist at Tecan Austria, Kevin Kopish is strategic marketing manager for cell analysis and J. Stevenson is general marketing manager at Promega, M. Salomon is vp development at Sirion Biotech, and M. Drewitz is senior research assistant and J.M. Kelm is co-founder and CSO at InSphero.



