November 1, 2008 (Vol. 28, No. 19)
Technique Leads to Advances in Assay Development and Cell Maintenance and Expansion
Cell-based assays are one of the most important techniques in drug discovery and toxicology screens, facilitating in vivo analysis of promising drug compounds or toxic agents. Researchers are increasingly adopting a cell-based approach to their drug discovery research, shifting their focus away from biochemical analysis of discrete cellular components to evaluate cellular function including tissue and organ activity through live cell imaging.
These types of assays—categorized as high-content screening (HCS)—allow researchers to study how drugs, proteins, or other biological materials affect cell viability, gene expression, and the respective signaling pathways. The utilization of living cells leads to more comprehensive data to strengthen results from biochemical and other assays used in the drug discovery processes.
During the last decade, drug discovery has become a fully integrated industrial process leading to an increasing need for cultivated cells and automated solutions for their production. This usage has resulted in the development of both products and systems designed to satisfy the stringent requirements for consistency, sterility, and quality demanded by cell-culture processes.
By replacing laborious and time-consuming manual processes, automation reduces costs, increases throughput, and enables round-the-clock production. Consistency and the high level of process flexibility provided by computer-based systems are still the primary reasons for the use of robotics. Thus applying automation to tissue-culture techniques, can improve assay development as well as cell maintenance and expansion.
Greiner Bio-One in collaboration with the Genomics Institute of the Novartis Research Foundation (GNF), developed the CellStar® AutoFlask™, a cell culture flask for automated tissue culture, increasing the range of applications in robotic systems.
The external dimensions correspond to ANSI Standards, making the flask suitable for use on a wide range of existing cell culture and liquid-handling systems. One of the design features of the AutoFlask is the built-in centrifugation pocket, which enables separation of cells from supernatant inside the flask.
Upon horizontal centrifugation, cells are collected inside the pocket whereas the medium remains in the bottom vessel of the flask. The robot-accessible precut multiple-entry septum enables sterile media and solution exchange and the removal of cell-based products. Precutting of the septum provides a number of benefits—it prevents coring by the injection needle, permits reclosing of the septum slit, and ensures sterility of the flask contents throughout the complete cultivation process.
The septum is located at the A1 position (of a 384-wellplate) to ease programming and piercing with any liquid-handling system. There is no need to move the flask into an upright position to access cells or media. Consequently the CellStar AutoFlask can remain in a horizontal position during the entire cultivation period to minimize any cell stress due to flask movement.
The integrated filter membrane facilitates gas exchange while providing a sterile barrier against contamination. The hydrophobic membrane is PTFE coated to prevent wetting of the filter from the internal flask contents. The venting area design together with the high airflow rate of the filter material provides an optimized oxygen supply that will allow the cultivation of even sensitive cells.
The table shows the impact of CellStar AutoFlask on specific cell line growth in comparison to other automation-friendly cell culture flasks. As growth areas of these flasks differ, the initial seeding concentrations were adjusted accordingly to ensure the evaluation was conducted on a like-for-like basis.
Vitality of SKNMC and CHO
Cultivation of SKNMC and CHO cells using CellStar AutoFlask as well as competitive automated cell culture flasks under the described settings led to comparable cell vitality values, indicating appropriate cell culture conditions for each experimental setup.
To analyze cell proliferation processes in more detail, a defined starting concentration of SKNMC and CHO cells was seeded in the respective media and maximum cell numbers were analyzed on each day of cultivation (Figure 1).

Figure 1. Vitality of SKNMC and CHO cells after 72 hours of cultivation.
CHO Growth Curve
Detailed analysis of CHO cell propagation revealed accelerated cell proliferation after the second day in vitro in the CellStar AutoFlask when compared to competitors 1 and 2. Maximum cell numbers dependent on the provided cell growth area were achieved between 72 and 96 hours of cultivation, whereas these were not achieved in the same timeframe using the flasks from competitors 1 and 2.
This indicates cell splitting and expansion can already be initiated after 72 hours when using CellStar AutoFlask, enabling a significant time saving throughout the entire cell-preparation process (Figure 2).
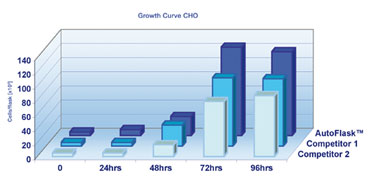
Figure 2. CHO cells were cultivated at 37°C and 5% CO2 for 96 hours.
SKNMC Growth Curve
SKNMC cells cultivated in CellStar AutoFlask demonstrated a similar effect as CHO cells, although less prominent due to a slower proliferation rate compared to CHO cells. Despite this inhibition in cell division, CellStar AutoFlask provided notable cultivation conditions leading to increased cell numbers after four days in vitro (Figure 3).
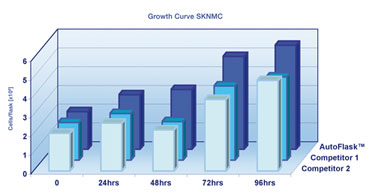
Figure 3. SKNMC cells were cultivated at 37°C and 5% CO2 for 96 hours.
Conclusion
Different experimental approaches using CellStar AutoFlask revealed optimal cell growth conditions for both common and sensitive cell lines leading to accelerated cell proliferation under these circumstances.
The CellStar AutoFlask offers a centrifugation pocket and an easy-to-access septum as well as good cultivation conditions assuring a reliable supply of cells with consistent quality in automated systems. It also offers an extensive range of applications and compatibility to various cell culture and liquid-handling systems to facilitate earlier cell harvest and time savings in the entire cultivation process.
Lara Marchetti, Ph.D. (Lara.Marchetti @gbo.com), is product manager R&D of cell culture products at Greiner Bio-One. Web: www.gbo.com/bioscience.



