August 1, 2009 (Vol. 29, No. 14)
Gyrolab System Designed to Circumvent the Problems Associated with Conventional Methods
The quantification of proteins is an integral part of biopharmaceutical R&D. From the crude quantitation of hybridoma expression profiles of monoclonal antibodies, to quantitative determination of biologic drugs and protein biomarkers in clinical trials, ligand binding assays have become a research standard. Low equipment cost requirements, sensitivity, and the fact that samples do not usually require substantial cleanup are the principle advantages.
Ligand binding assays, however, can be time consuming, suffer from matrix effects, and be challenging to validate to current ICH/FDA guidelines. A reduction in analysis time, sample volume, and time to transfer and validate assays would provide a significant advantage to the progression of biopharmaceutical portfolios within the industry. We recently evaluated the Gyrolab® from Gyros, a semi-automated, low-volume assay system, for its ability to address these issues.
Assay Explanation
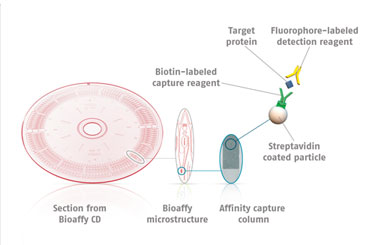
Instead of performing the assay in microtiter plates, Gyrolab system uses injection-molded microfluidic channels on the surface of a plastic disc, identical in dimensions to the standard compact disc (CD). Liquids are added to microstructures by a robotic microfluidic handling system. By a combination of capillary action, hydrophobic barriers, and centrifugal force (produced by spinning the disc), liquids are delivered to desired parts of each microstructure (Figure 1).
Each microstructure contains a Streptavidin-conjugated bead resin column, 15 nanoliters in volume. Step-wise addition of biotinylated capture reagent, samples, and then fluorophore-labeled detection reagent forms the classic sandwich assay. The fluorescent signal on each column is then read upon excitation by the red laser. Alexa Fluor® 647 (Invitrogen/Life Technologies) is commonly employed as the detection label, although other dyes with similar spectral properties can also be used, e.g., DyLight™ 649 (Thermo Fisher Scientific).
The use of such fluorophores facilitates the possibility of generating a sensitive assay using small sample volumes. There are three different discs that one can use to define different sample volumes applied to the affinity-capture columns in the micro-structures. Each disc contains sample definition chambers above the affinity column of either 20 nL, 200 nL, or 1,000 nL in volume.
Therefore, for sample analysis where the analyte of interest is present in high concentrations, i.e., 10 µg/mL or greater, the 20 nL CD should be used, to effectively shift the working range of the assay. For analysis of biomarkers, whose likely concentration in a sample is in the order of 50 pg/mL, the 1,000 nL CD is more effective, as it ensures a larger amount of sample is exposed to the affinity column.
Calibration standards, QC samples or unknown sample replicates are analyzed in individual microstructures. Eight microstructures are contained within each segment. They are connected to common reagent channels for the application of capture and detection reagents within the same segment. As a result, it is possible to run different assays on different segments of a disc (i.e., reagent screening applications).
As each microstructure is processed in parallel with all others on the disc, the time required to run an assay on 112 data points (capacity of both 20 nL and 200 nL CDs, 96 data points on the 1,000 nL CD) is just under one hour. This provides a time saving of at least two hours over conventional immunoassays. In addition, as the assay is automated, no further operator input is required once the disc and samples are loaded into the machine.
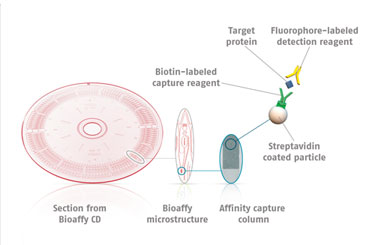
Figure 1. The Bioaffy® CD platform on which assays are performed
Quality
Ligand binding assays can be subject to a number of variables that occur during the time course of the experiment. Operator pipetting technique, pipette performance, evaporation during long incubation periods, temperature-driven edge effects, and sporadic coating are some of the issues that must be controlled for assays to fall within FDA best practices guidance for ligand binding assays during validation.
In most cases, robotic liquid-handling systems tend to reduce imprecision in replicates over that observed in manual pipetting manipulations. With the Gyrolab workstation, we commonly observe levels of imprecision no greater than 5%. This falls well within the 20% imprecision industry-wide acceptance criteria for ligand binding assays. The most common reason for the criteria to fail in a Gyrolab experiment is when particulate matter is present in samples; simply preparing samples and reagents by centrifugation or filtration alleviates this issue.
A crucial parameter in successful assay development is the quality and affinity of the assay reagents, i.e., the antibodies used. This holds true for standard ligand binding assay development, but affinity is all the more crucial in Gyrolab assays, as the contact time across the affinity column in each microstructure is approximately six seconds. Thus, reagents with slow on-rates perform poorly, leading to the need for high affinity, fast on-rate antibodies. It is common to purchase reagents that are either high-affinity monoclonal antibodies, or affinity-purified polyclonal antibodies.
Method Development
One of the main advantages of the Gyrolab assay is the speed in which assay parameters can be screened. This is beneficial as an operator often needs to screen and optimize a number of variables in an experiment to rapidly generate a fit-for-purpose assay.
Four detector antibodies and four capture antibodies were screened for performance in the method development of a bioanalytical assay for a biopharmaceutical (Figure 2). Overall, ten different conditions were employed on one disc. Within an hour, the assay demonstrated an optimal pair of antibodies, with a broad linear range. This was further confirmed by inspection of the individual column fluorescence profiles produced during the assay.
These profiles reflect the innate affinity of the reagents for the analyte, where a sharp peak located at the top of the column indicates a high-affinity interaction. This approach can guide users as to which reagents they should employ and could also be used to assess affinity in other situations, e.g., hybridoma supernatants screening for monoclonal antibodies against a particular antigen.
During method development, ligand binding assays are often characterized for their susceptibility to biological matrix effects (detection of the analyte of interest is often required from complex biological matrices such as plasma or serum) by linearity of dilution experiments. Most of these effects are driven by low-affinity, nonspecific interactions between reagents/analyte and endogenous components within the sample. Such effects are enhanced by prolonged incubation times, and again the Gyrolab assay provides an advantage. Due to the short contact time of samples with the affinity column, such nonspecific interactions are minimized and are often ablated completely.
A current limitation is the inability to perform multiplexing reactions where there is a requirement to analyze multiple analytes in one sample. For single analyte measurement, however, Gyrolab certainly provides advantages to a laboratory over other conventional techniques.
Ligand binding assays continue to be employed in the bioanalysis of biopharmaceuticals in the industry, but time to develop, transfer, and run assays can often slow programs down. Automated, high-quality systems provide clear advantages for the development of biologics, and Gyrolab aids in the development of high-quality, low sample volume, reagent saving, rapid assays that are readily transferred to other sites with the technology, e.g., CROs.
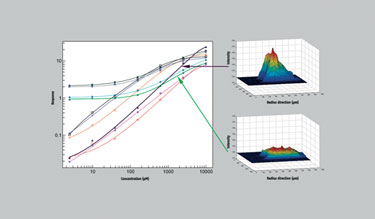
Figure 2. Comparison of 10 combinations of reagents used in the development of a mAb quantitation assay.
Kevin Brady ([email protected]) is principal scientist in the pharmacokinetics, dynamics, and metabolism division at Pfizer Global Research and Development.