Vaxxas opened its first manufacturing facility in Brisbane, Queensland. The 5,500 m2 (60,000 ft2) Vaxxas Biomedical Facility will serve as the company’s global headquarters and support the scaleup of its operations to produce HD-MAP vaccines for future late-stage clinical trials and first commercial products.
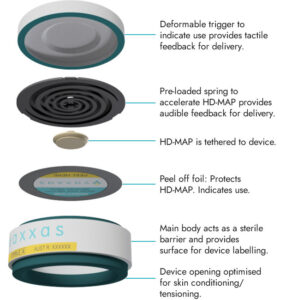
The company’s HD-MAP technology platform relies on an ultra-high-density array of projections—invisible to the naked human eye—applied to the skin as a patch sitting inside a small applicator device. When applied to the skin, the patch delivers vaccine to the immune cells immediately below the skin surface. This approach can enhance the efficiency and effectiveness of resulting immune responses of vaccines, according to the company.
Dry-coating technology
Vaxxas uses proprietary dry-coating technology to apply an active and stable vaccine onto the projections, which offers the potential to eliminate the need for vaccine refrigeration during storage and transportation, thus reducing the resource and logistics burden of maintaining the refrigerated cold chain, notes David Hoey, Vaxxas’ CEO, who adds that ease of use of the HD-MAP could enable simplified administration, potentially encompassing self-administration.
Vaxxas’ HD-MAP delivered vaccines are under investigation and available only for investigational uses. They are not available anywhere in the world for sale or purchase.


