Researchers at Cold Spring Harbor Laboratory say they have discovered a mechanism that could lead to a drug that only affects the cells and neurons involved in the treatment of strokes and seizures without unwanted side effects.
According to Hiro Furukawa, PhD, a professor at Cold Spring Laboratory, “it really comes down to chemistry.”
When the human brain is injured, such as during a stroke, parts of the brain begin to acidify. This acidification leads to the rampant release of glutamate.
“We suddenly get more glutamate all over the place that hits the N-methyl-D-aspartate (NMDA) receptor and that causes the NMDA receptor to start firing quite a lot,” explained Furukawa, who oversaw the study (“Structural elements of a pH-sensitive inhibitor binding site in NMDA receptors”), which appeared recently in Nature Communications.
“Context-dependent inhibition of NMDA receptors has important therapeutic implications for the treatment of neurological diseases that are associated with altered neuronal firing and signaling. This is especially true in stroke, where the proton concentration in the afflicted area can increase by an order of magnitude. A class of allosteric inhibitors, the 93-series, shows greater potency against GluN1-GluN2B NMDA receptors in such low pH environments, allowing targeted therapy only within the ischemic region,” wrote the investigators.
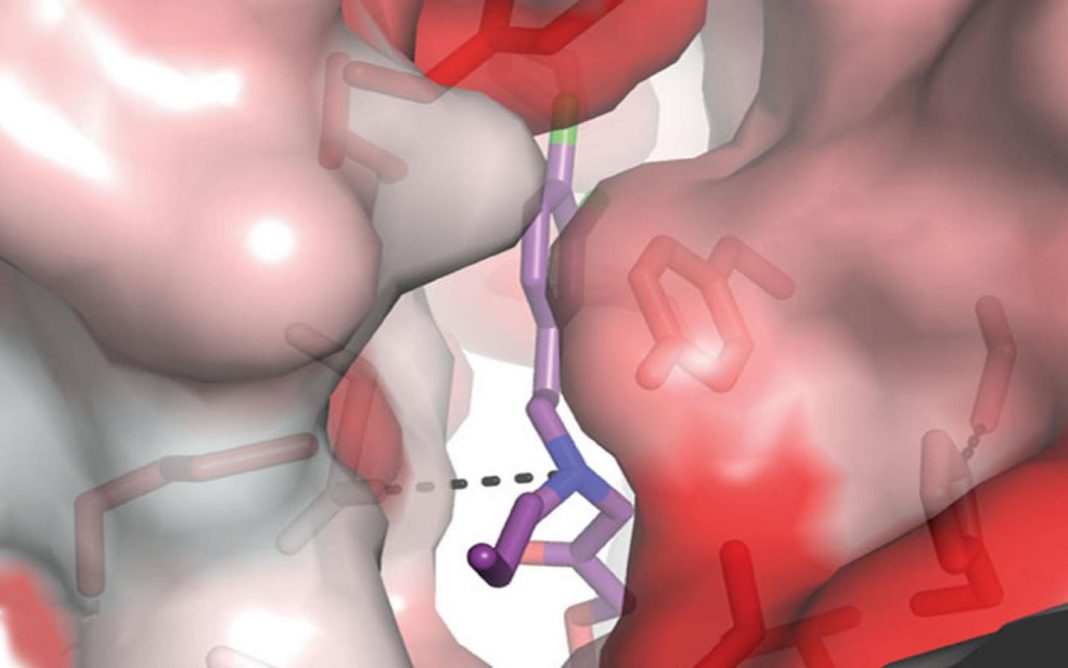
“Here we map the 93-series compound binding site in the GluN1-GluN2B NMDA receptor amino terminal domain and show that the interaction of the N-alkyl group with a hydrophobic cage of the binding site is critical for pH-dependent inhibition. Mutation of residues in the hydrophobic cage alters pH-dependent potency, and remarkably, can convert inhibitors into potentiators. Our study provides a foundation for the development of highly specific neuroprotective compounds for the treatment of neurological diseases.”
In a healthy brain, the NMDA receptor is responsible for controlling the flow of ions in and out of a neuron. The “firing” of these signals is crucial for learning and memory formation. However, overactive neurons can lead to disastrous consequences. Abnormal NMDA receptor activities have been observed in various neurological diseases and disorders, such as stroke, seizure, depression, and Alzheimer’s disease, and in individuals born with genetic mutations.
Furukawa’s team, in collaboration with scientists at Emory University, looked for a way to prevent over-firing NMDA receptors without affecting normal regions of the brain.
Previous work had identified promising compounds, called the 93-series, suited to this purpose. Eager to join with the NMDA receptor in an acidic environment, these compounds downregulate the receptor activity, even in the presence of glutamate, thereby preventing excessive neuronal firing.
However, the 93-series compounds sometimes cause the unwanted consequence of inhibiting the NMDA receptors in healthy parts of the brain. That’s why Furukawa and his colleagues set out to determine how they could improve upon the unique features of the 93-series.
Using x-ray crystallography, the researchers were able to see that a motif on the 93-series compound slots into a tiny, never-before-noticed pocket within the NMDA receptor. Experimentation showed that this pocket is particularly sensitive to the pH around it.
“Now that we see the pH-sensitive pocket within NMDA receptors, we can suggest a different scaffold,” Furukawa explained. “We can redesign the 93-series chemical compound—let’s call it 94-series—in such a way that it can more effectively fit to that pocket and a higher pH sensitivity can be obtained. So, we’re basically just starting our effort to do that.”