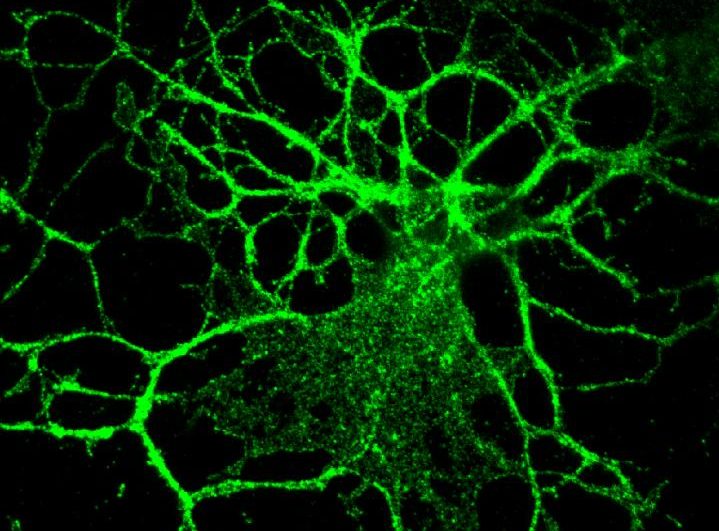
A team of researchers at Case Western Reserve University (CWRU) have developed a potential treatment strategy for Pelizaeus-Merzbacher disease (PMD). Using a mouse model, the team identified a toxic protein as a therapeutic target and used a family of drugs known as antisense oligonucleotides (ASOs) to target the RNA strands that created the abnormal protein to stop its production.
Their study, “Suppression of proteolipid protein rescues Pelizaeus-Merzbacher disease,” was recently published in Nature and was led by Paul Tesar, PhD, professor in the department of genetics and genome sciences at the CWRU School of Medicine and the Dr. Donald and Ruth Weber Goodman professor of innovative therapeutics.
PMD is a rare, progressive, degenerative central nervous system disorder in which coordination, motor, and intellectual function deteriorate. The disease is one of a group of gene-linked disorders called leukodystrophies that affect the growth of the myelin sheath—sleeves of fatty tissue that protect your nerve cells and promote the rapid transmission of nerve impulses—and primarily affects children. The signs of the classical form of PMD usually beings during early infancy. PMD is caused by the inability to form myelin due to a defect in the proteolipic protein (PLP1) gene.
After identifying the toxic protein, PLP1, as a treatment target and using ASOs to stop its production, PMD’s symptoms were reduced and lifespan was expanded.
“To evaluate the translational potential of this strategy we identified ASOs that stably decrease Plp1 mRNA and protein throughout the neuraxis, in vivo. Administration of a single dose of Plp1-targeting ASOs to postnatal jimpy mice fully restored oligodendrocyte numbers, increased myelination, improved motor performance, normalized respiratory function, and extended lifespan through an eight-month endpoint. These results support the development of Plp1-suppression as a treatment for PMD,” wrote the researchers.
“The preclinical results were profound. PMD mouse models that typically die within a few weeks of birth were able to live a full lifespan after treatment,” said Tesar. “Our results open the door for the development of the first treatment for PMD as well as a new therapeutic approach for other myelin disorders.”
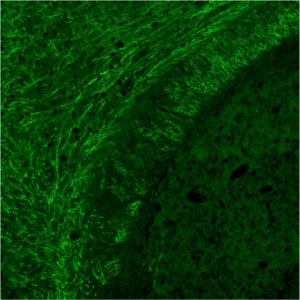
By demonstrating effective delivery of the ASOs to myelin-producing cells, researchers raised the prospect for using this method to treat other myelin disorders such as multiple sclerosis (MS).
“ASOs provided an opportunity to cut the disease-causing protein off at its source,” noted Matthew Elitt, who worked in Tesar’s lab as a Case Western Reserve medical and graduate student.
ASOs are short single-stranded polymers based on DNA or RNA chemistries that are chemically synthesized. They can regulate gene expression by binding in a sequence-specific manner to an RNA target. They are considered a relatively new class of drugs. In 2016, the FDA approved the first ASO drug for a neurological disorder, spinal muscular atrophy. Multiple ASOs with different clinical applications have entered the market. However, some ASOs have failed to enter the market due to reasons including limited added clinical benefit, systemic safety concerns, and costs of manufacturing. Yet recent developments seem to hold promise.
Tesar noted that ongoing and planned experiments in his laboratory will help aid future clinical development of ASO therapy for PMD.
“While important research questions remain, I’m cautiously optimistic about the prospect for this method to move into clinical development and trials for PMD patients,” Tesar explained. “I truly hope our work can make a difference for PMD patients and families.”