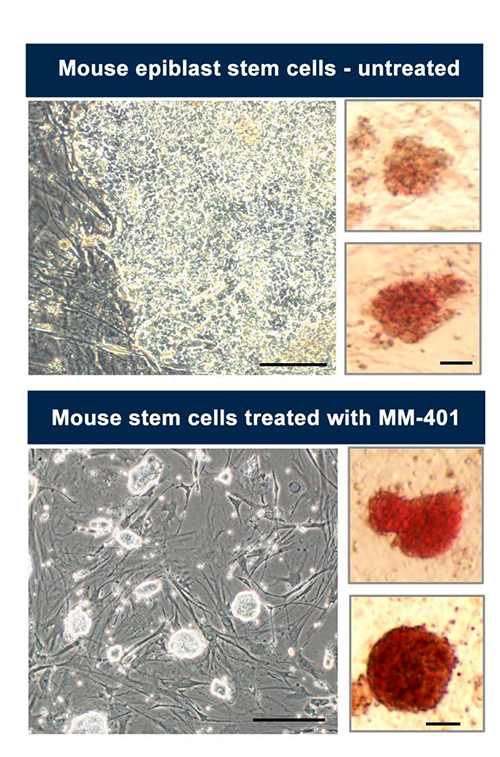
Mouse epiblast stem cells that have already begun the journey to differentiating into “adult” cells are shown in the upper image. But when the same kind of cells were treated with a drug called MM-401, they reverted to a naïve pluripotent state, as shown in the lower image. [University of Michigan]
A drug originally intended to treat leukemia has an unexpected power. It can reverse stem cell development, converting primed pluripotent stem cells to naïve pluripotent stem cells. The drug, called MM-401, effectively removes epigenetic markers from histones, depriving the cell’s DNA-reading machinery of indications of where to start reading. Stripped of its accumulated “Post-it notes,” the DNA instruction manual is like new. It lacks any indications that any sections should receive any special attention.
The surprising finding comes from work performed at the University of Michigan School of Medicine. Here, scientists found that more than half of mouse epiblast stem cells treated with MM-401 reversed course within 3 days and regained an embryonic “be anything” state, also called pluripotency. In addition to generating pluripotent stem cells, the scientists showed that mice bred using the cells grew up healthy.
The scientists, led by Yali Dou, Ph.D., presented their results March 17 in the journal Cell Stem Cell, in an article entitled, “MLL1 Inhibition Reprograms Epiblast Stem Cells to Naive Pluripotency.” The article describes how MM-401 was used to block histone H3K4 methyltransferase MLL1 activity. This epigenetic perturbation, the article indicates, sufficed to initiate an epiblast stem cell (EpiSC) to embryonic stem cell (ESC) reversion.
“This reversion is highly efficient and synchronized, with more than 50% of treated EpiSCs exhibiting features of naive ESCs within 3 days,” wrote the article’s authors. “Reverted ESCs reactivate the silenced X chromosome and contribute to embryos following blastocyst injection, generating germline-competent chimeras.”
The authors added that blocking the MLL1 gene leads to global redistribution of the histone H3K4me1 at enhancers and represses lineage determinant factors and EpiSC markers, which indirectly regulate ESC transcription circuitry.
“We've demonstrated that we don't have to manipulate the pluripotent genes to get to the ground state, but rather that we can block all other options of where the cell 'wants' to go,” said Dr. Dou. “Then the only option is going back to the ground, or naïve, pluripotent state.”
MLL1 plays a key role in the uncontrolled explosion of white blood cells that's the hallmark of leukemia, which is why University of Michigan researchers originally developed MM-401 to interfere with it. But it also plays a much more mundane role in regular cell development and in the formation of blood cells and the cells that form the spinal cord in later-stage embryos.
It does this by placing methyl groups on histones. Without those labels, the cell's DNA-reading machinery doesn't know where to start reading. It's as if the invitation to open the instruction manual had vanished.
Stem cells don't harness the power of MLL1 until they're older. So using MM-401 to block MLL1's normal activity in cells that had started down the path to adulthood meant that histone marks were missing before the cell needed them. The cells couldn't continue on their journey to becoming different types of cells. But they could still function as healthy pluripotent stem cells.
“People have been focused on other epigenetic changes that are more dramatic, but ignored methylation by the MLL family,” noted Dr. Dou. “Deleting MLL1 entirely causes failure later in differentiation. But inhibiting it with a drug temporarily leaves no trace behind.”



