New research has uncovered how a dietary intervention could help prevent the development of the autoimmune disease, systemic lupus erythematosus (SLE). Using mouse models of lupus, the team from Yale University set out to test the role of diet and the microbiota and dissect its mechanisms as the role of commensal bacteria in autoimmunity remains unclear.
“We dissected, molecularly, how diets can work on the gut microbiome,” said senior author Martin Kriegel, M.D., Ph.D., associate professor adjunct in the department of immunobiology, Yale University School of Medicine. “We identified a pathway that is driving autoimmune disease and mitigated by the diet.”
The paper, A Diet-Sensitive Commensal Lactobacillus Strain Mediates TLR7-Dependent Systemic Autoimmunity was published recently in Cell Host & Microbe.
The team first identified the bacterium, Lactobacillus reuteri, in the gut of the mice that triggered an immune response leading to the disease. Specifically, in the Toll-like receptor 7 (TLR7)-dependent mouse models of lupus, L. reuteri stimulated immune cells known as dendritic cells, as well as immune system pathways that exacerbated disease development.
To investigate the potential impact of diet on the presence of this bacteria in the mice, first author Daniel Zegarra-Ruiz, a graduate student in the lab, fed the mice “resistant starch”— a diet that mimics a high-fiber diet in humans. Foods that are high in resistant starch are rice, whole grains such as oats and barley, beans, peas, and lentils.
The resistant starch is not absorbed in the small intestine but ferments in the large intestine, enriching good bacteria and causing the secretion of short-chain fatty acids. The diet suppressed both the growth and movement of L. reuteri bacteria outside the gut that would otherwise lead to autoimmune disease.
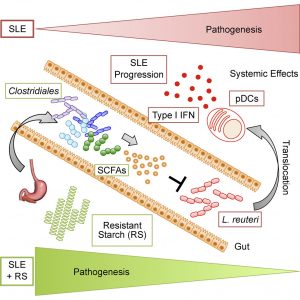
In addition to the finding that L. reuteri can drive autoimmunity but is ameliorated by dietary resistant starch (RS), cultures of internal organs and 16 S rDNA sequencing revealed that the L. reuteri had spread to the intestines, liver, and spleen in the mouse model of lupus but not in healthy mice.
SLE, like other autoimmune diseases, is characterized by a type I interferon signature mediated by pDCs. L. reuteri colonization worsened autoimmune manifestations under specific-pathogen-free and gnotobiotic conditions, increasing plasmacytoid dendritic cells (pDCs) and interferon signaling.
However, the RS diet suppressed the abundance and translocation of L. reuteri via short-chain fatty acids, which inhibited its growth. Additionally, RS decreased pDCs, interferon pathways, organ involvement, and mortality, exerting beneficial effects in lupus-prone mice through suppressing bacteria that promote interferon pathways implicated in the pathogenesis of human autoimmunity.
Western lifestyle is linked to various inflammatory conditions such as autoimmune and metabolic diseases, driven by changes in diet and gut microbial composition, as suggested by the hygiene hypothesis. Gut microbiota and their metabolites mediate mucosal and systemic immune responses, and alterations in its composition have been linked to multiple autoimmune disorders.
The study also found an imbalance of gut microbes in a subset of lupus patients that was similar to what they observed in lupus-prone mice not given the starch diet. In this subset of lupus patients, the high-fiber diet could potentially be beneficial to prevent or ameliorate the condition, in addition to other diseases that activate the same immune pathway, Dr. Kriegel noted. “It may have implications beyond lupus.”
While more research is needed to discern how the findings translate to humans, the study details an important link between diet, gut bacteria, and autoimmunity







