Mutations in a calcium ion channel anoctamin 5 (ANO5), commonly found in muscle cells, cause the debilitating disease called limb girdle muscular dystrophy 2L (LGMD2L). Characterized by progressive adult-onset muscle weakness, LGMD2L patients are often asked to “keep mobile” with no specific guidelines on the type or intensity of activities but weakness in muscles of the calves, thighs, and arms makes it difficult for patients to tolerate physical activity.
“There are no therapies available,” said Jyoti Jaiswal, PhD, principal investigator at Children’s National Hospital’s Center for Genetic Medicine Research.
In an article titled, “Endoplasmic reticulum maintains ion homeostasis required for plasma membrane repair,” published in the Journal of Cell Biology, Jaiswal and his lab identifies a calcium pump (SERCA) in the endoplasmic reticulum (ER)—an interconnected multifunctional network of membrane-bound tunnels that constitutes the expressway in the cell—that together with ANO5, funnels calcium ions into the tunnels of the ER and helps allay the pathological effects of the surge of calcium ions into the cell when its outer membrane (plasma membrane) is injured.

“This study opens the path for developing targeted therapies for LGMD2L and provides a fundamental cellular insight into a process crucial for cell survival,” said Goutam Chandra, PhD, postdoctoral research fellow in Jaiswal’s lab and first author on the study.
“The study provides a novel insight into how injured cells in our body cope with calcium ion imbalance during injury,” said Jaiswal. “This work also addresses how calcium homeostasis is compromised by a genetic defect that leads to LGMD2L. It also offers a proof-of-principle approach to restore calcium homeostasis, paving the path for future work to develop therapies targeting this disease.”
These results address the current lack of understanding of the basis for exercise intolerance and other symptoms faced by LGMD2L patients, Jaiswal said.
ANO5 supports SERCA’s calcium ion import into the lumen of the ER by simultaneously importing negatively charged ions (anions) into the ER to balance the positive charge on incoming calcium ions, so that the environment within the ER stays electrically neutral.
Malfunction of either ANO5 or SERCA can result in calcium ion overload in the cytosol, disrupting processes of plasma membrane repair (PMR) critical in repairing muscle fibers that are prone to injury during physical activity.
The concentration of calcium ions is nearly a thousand-fold higher outside the cell than in the cytosol. This steep slope is maintained by the calcium channels that mobilize calcium ions into the extracellular space or into compartments within the cell, such as the ER. Mutations in ANO5 in the muscle fibers of LGMD2L patients compromises their ability to regulate the physiological balance of calcium ions in the cytosol and ER.
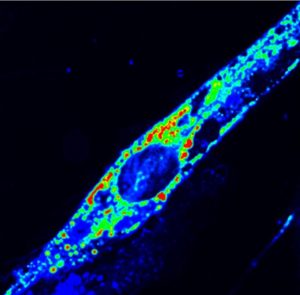
However, the authors reported, whereas muscle cells from healthy controls can regain normal levels of cytosolic calcium ion concentrations, muscle cells that are ANO5-deficient continue to harbor high levels of calcium ion in their cytosols and are not able to repair their plasma membranes.
Toward identifying a therapeutic strategy, the authors then tested whether pharmacologically reducing the calcium ion concentration in the cytosol of muscle cells improves the ability of ANO5-deficient muscle cells to repair their plasma membranes. The authors showed that treating mutant muscle cells with a potent calcium ion chelator (compound that binds to calcium ions) improved their ability to repair the injured plasma membrane.
Commenting on whether there are existing drugs that are blockers of Ca ion transport that can be repurposed to treat LGMD2L patients or whether new drugs will need to be developed based on these findings Jaiswal said: “There are several calcium channel blockers in use, but their utility for this indication has not been tested as yet.”
This study aimed at understanding how cells repair from injury has revealed a hitherto unknown role of the ER in facilitating PMR in injured cells.



