Researchers at the Lewis Katz School of Medicine at Temple University (LKSOM) say they have uncovered a novel mechanism by which abnormalities in mitochondrial fission in endothelial cells contribute to inflammation and oxidative stress in the cardiovascular system. They further show how the fission-fusion balance can be stabilized to lower inflammation using salicylate, the main active ingredient in everyday pain-relieving drugs like aspirin. Their study “Mitochondrial Fission Mediates Endothelial Inflammation” appears in Hypertension.
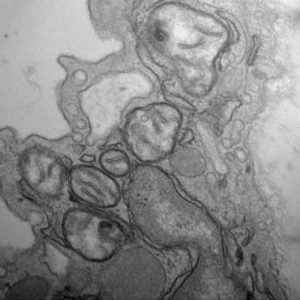
“Endothelial inflammation and mitochondrial dysfunction have been implicated in cardiovascular diseases, yet a unifying mechanism tying them together remains limited. Mitochondrial dysfunction is frequently associated with mitochondrial fission/fragmentation mediated by the GTPase Drp1 (dynamin-related protein 1). Nuclear factor (NF)- kB, a master regulator of inflammation, is implicated in endothelial dysfunction and resultant complications. Here, we explore a causal relationship between mitochondrial fission and NF-kB activation in endothelial inflammatory responses. In cultured endothelial cells, TNF-a (tumor necrosis factor-a) or lipopolysaccharide induces mitochondrial fragmentation. Inhibition of Drp1 activity or expression suppresses mitochondrial fission, NF-kB activation, vascular cell adhesion molecule-1 induction, and leukocyte adhesion induced by these proinflammatory factors,” write the investigators.
“Moreover, attenuations of inflammatory leukocyte adhesion were observed in Drp1 heterodeficient mice as well as endothelial Drp1 silenced mice. Intriguingly, inhibition of the canonical NF-kB signaling suppresses endothelial mitochondrial fission. Mechanistically, NF-kB p65/RelA seems to mediate inflammatory mitochondrial fission in endothelial cells. In addition, the classical anti-inflammatory drug, salicylate, seems to maintain mitochondrial fission/fusion balance against TNF-a via inhibition of NF-kB. In conclusion, our results suggest a previously unknown mechanism whereby the canonical NF-kB cascade and a mitochondrial fission pathway interdependently regulate endothelial inflammation.”

“It was already known that in cardiovascular disease the function of endothelial cells and mitochondria are impacted by inflammation, but we were unsure whether there was a link between the two,” explained Satoru Eguchi, MD, PhD, Professor of Physiology and Professor in the Cardiovascular Research Center, Sol Sherry Thrombosis Research Center, and Center for Metabolic Disease Research at LKSOM.
In endothelial cells, chronic inflammation causes mitochondria to become smaller and fragmented. This damaging process is mediated by a molecule known as dynamin-related protein 1 (Drp1). Normally, Drp1 plays a helpful role in maintaining fission-fusion balance. When cells are stressed by inflammation, however, it steps up fission activity, resulting in mitochondrial fragmentation.
“How Drp1 acts to increase mitochondrial fragmentation when endothelial cells are inflamed has been unclear,” Eguchi said. “But we wondered whether it might interact with nuclear factor (NF)- kB, which oversees the regulation of inflammatory processes and is involved in endothelial dysfunction.”
In endothelial cells, Eguchi and colleagues stimulated inflammatory pathways that produced mitochondrial fragmentation. They then examined the effects of blocking Drp1 activity and expression. These experiments showed that in cells, Drp1 inhibition suppresses mitochondrial fission, NF-kB activation, and inflammation. Reductions in fission and inflammation were also observed in cells following NF-kB inhibition, as well as in follow-up studies in mice genetically engineered to have less Drp1.
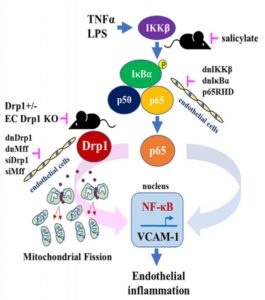
The researchers next determined whether the anti-inflammatory drug salicylate could also reduce mitochondrial fragmentation. Salicylate works by blocking the activity of multiple inflammatory molecules, including NF-kB. As anticipated, in mice, treatment with salicylate attenuated inflammation and mitochondrial fragmentation via its effects on NF-kB and downstream pathways.
“Our findings suggest that salicylate may be able to maintain the balance between mitochondrial fission and fusion under inflammatory conditions,” Eguchi said. “This observation could have real clinical impact, since salicylate is already used in aspirin and related pain-relievers.”
In future work, Eguchi plans to explore the influence of aging and other factors on Drp1 and mitochondrial fission in endothelial cells.
“Mitochondrial function declines with aging, but we also know that exercise and diet influence this process. How these factors come together mechanistically to impact vascular health is not fully understood,” Eguchi explained.