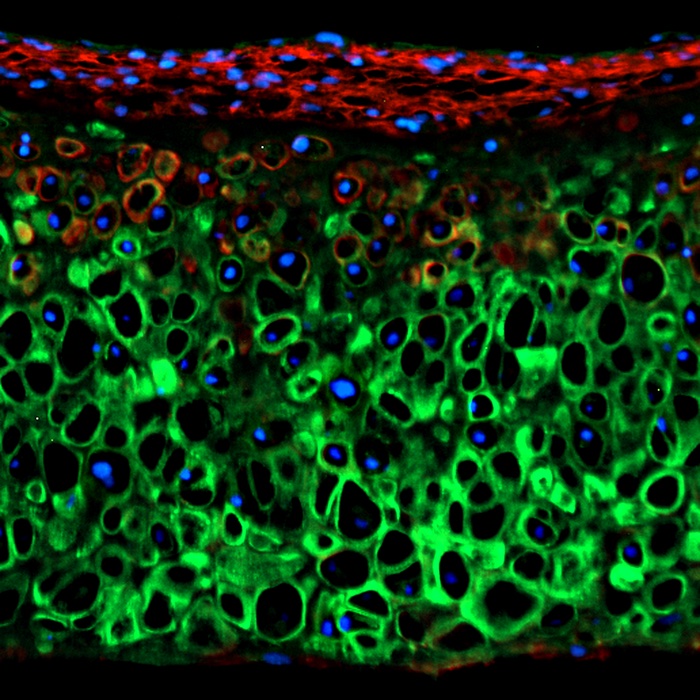
Cartilage is the tough but flexible tissue that covers the ends of your bones at a joint. It also gives shape and support to other parts of your body. Injured, inflamed, or damaged cartilage can cause symptoms such as pain and limited movement. It can also lead to joint damage and deformity. A new study by researchers at Cytex Therapeutics and Washington University may lead to more effective treatments for osteoarthritis and other cartilage diseases, as well as hereditary disorders affecting cartilage development.
The findings are published in the journal Stem Cells, in a paper titled, “Transient receptor potential vanilloid 4 as a regulator of induced pluripotent stem cell chondrogenesis.”
The researchers demonstrated that a gene, transient receptor potential vanilloid 4 (TRPV4), serves as a marker and a regulator of chondrogenesis—the process by which cartilage and bone are formed.
“TRPV4 is a polymodal calcium-permeable cation channel that is highly expressed in cartilage and is sensitive to a variety of extracellular stimuli,” wrote the researchers. “The expression of this channel has been associated with the process of chondrogenesis in adult stem cells as well as several cell lines. Here, we used a chondrogenic reporter (Col2a1-GFP) in murine induced pluripotent stem cells (iPSCs) to examine the hypothesis that TRPV4 serves as both a marker and a regulator of chondrogenesis.”
“We wanted to learn whether TRPV4 is specifically expressed as a marker of chondrogenesis, and also if it plays a functional role in regulating chondrogenesis in iPSC-derived chondrocytes—the cells responsible for cartilage formation,” explained Farshid Guilak, PhD, from the Center of Regenerative Medicine at Washington University and corresponding author.
The researchers used a fluorescent indicator to monitor chondrogenic differentiation from murine (mouse) iPSCs. TRPV4 expression mirrored that of classic chondrogenic markers produced naturally by the body after a 21-day period.
“Furthermore, daily activation of TRPV4 significantly increased cartilage matrix production,” Guilak said.
“These findings tell us that, as we suspected, TRPV4 serves as both a marker and a regulator of iPSC chondrogenesis,” he added. “We believe that iPSC chondrogenesis could be an excellent model system for studying TRPV4’s role in cartilage development and diseases. An improved understanding of how this occurs promises to provide new insights into the development of new therapeutic approaches for cartilage-related disorders.
“Additionally, it could lead to a new way of optimizing stem cell differentiation when bioengineering cartilage designed for knee replacements and other joint repairs.”
This study demonstrates that using the role of TRPV4 in chondrogenesis may also provide a novel approach for accelerating stem cell differentiation in functional tissue engineering of cartilage replacements for joint repair.