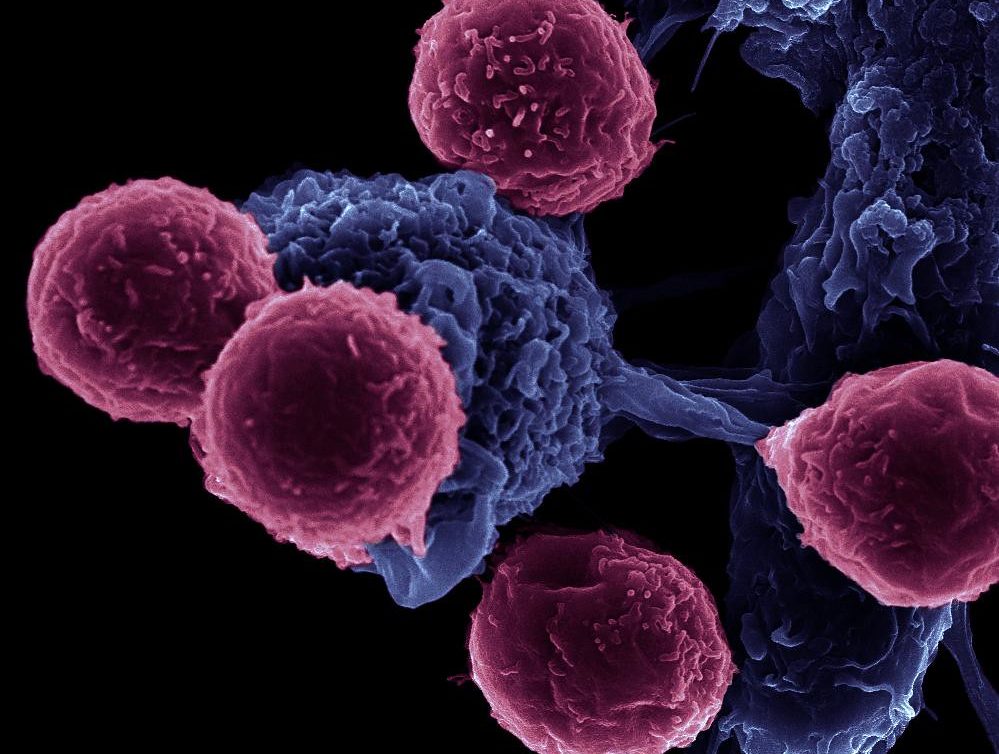
A new study provides an explanation as to why cancer immunotherapy currently fails in many patients and offers a potential solution that could increase the efficacy of anticancer drugs. Currently, 70 to 85% of patients taking immunotherapy drugs fail to respond to them.
In the study conducted on laboratory mice, Kazukani Hayashi, PhD, and a team of cancer researchers at Cedars-Sinai, showed that combining the chemotherapy drug, Gemcitabine, with the anti-inflammatory drug, Celecoxib (Celebrex), allowed the cancer drug to trigger an immune response, thereby increasing its efficacy. Gemcitabine is commonly prescribed to treat several solid tumors, including bladder and pancreatic cancer.
This work is published in Nature Communications, in an article titled, “Tipping the immunostimulatory and inhibitory DAMP balance to harness immunogenic cell death.”
Keith Syson Chan, PhD, a Cedars-Sinai Cancer translational scientist and corresponding author on the study, said this combination of drugs delivered a “one-two punch” by killing tumor cells and simultaneously activating immune cells.
“I believe that our study has significant clinical potential, as cancer immunotherapy continues to emerge as an important pillar for treating cancer patients,” Chan said. “This discovery, if confirmed in clinical trials, may potentially increase the percentage of patients who respond to cancer immunotherapy.”
The traditional modus operandi in cancer treatment has been to use chemotherapeutic drugs that kill cancer cells directly. These drugs, in most cases, suppress the patients’ immune response. Rarely do they engage the patient’s own immunity to compound their battle against cancer. Recently, immunotherapy has been used in conjunction with chemotherapy, or as a separate approach, to stimulate the patient’s own immune response, but the response rate is low.
Immunogenic cell death (ICD), inducible by drugs, can propagate anti-tumor immunity by deploying dendritic cells, professional immune cells, to catalyze CD8 positive T lymphocytes to destroy cancer cells. The trigger for ICD activation is a group of proteins released by dying cancer cells called damage-associated molecular patterns (DAMPs). To date, only a handful of anticancer drugs are bona fide ICD activators.
Initially believed to stimulate the patient’s own immune response against cancer cells through the release of triggering signals, Gemcitabine and other chemotherapeutic drugs simultaneously release inhibitory signals, preventing dendritic cells from activating T cells. Therefore, explained Chan, “the T cells don’t go anywhere.”
Adopting an unbiased proteomics approach, the authors demonstrate that Gemcitabine alone can indeed induce the release of DAMPs in both human and mouse models of bladder cancer and in a mouse pancreatic ductal adenocarcinoma (PDAC) model. However, in addition, Gemcitabine also triggers the release of prostaglandin E2 that inhibits DAMPs.
The balance of activating and inhibiting signals must be tipped in favor of activation for Gemcitabine to mount an effective immune response.
The investigators of the study, including researchers from Cedars-Sinai, Baylor College of Medicine in Houston, and Taipei Medical University in Taiwan, discovered that the anti-inflammatory drug Celecoxib was able to favorably adjust this critical balance.
Celecoxib effectively removes the inhibitory signals so that only the activating signals remain. In the presence of Celecoxib, Gemcitabine-activated dendritic and T cells could mount a successful immune response, converting Gemcitabine into an immunogenic chemotherapeutic drug.
Celecoxib, a selective Cox-2 molecular inhibitor, prevents the biosynthesis of prostaglandin E2, thereby preventing Gemcitabine from inhibiting DAMP-triggered ICD.
“Rather than focusing on stepping down harder on the gas pedal—releasing proteins that are “go” signals—we removed the impeding brake pedal, allowing the dendritic cells to induce T cells to kill tumors,” Hayashi said.
Adding a third component in the form of an immunotherapy drug may further increase the efficacy of the Gemcitabine and Celecoxib combined treatment, Chan and Hayashi believe. Experiments are currently ongoing in Chan’s lab to test this hypothesis and will be followed up with randomized, placebo-controlled human trials to test the efficacy of this new treatment paradigm.