Scientists at the University of Basel report the development of a precisely controllable system for mimicking biochemical reaction cascades in cells. Using microfluidic technology, the team produces miniature polymeric reaction containers equipped with the desired properties.
This “cell on a chip” is useful not only for studying processes in cells, but also for the development of new synthetic pathways for chemical applications or for biological active substances in medicine, according to the researchers, who published their study “Combinatorial Strategy for Studying Biochemical Pathways in Double Emulsion Templated Cell‐Sized Compartments” in Advanced Materials.
“Cells rely upon producing enzymes at precise rates and stoichiometry for maximizing functionalities. The reasons for this optimal control are unknown, primarily because of the interconnectivity of the enzymatic cascade effects within multi‐step pathways,” write the investigators.
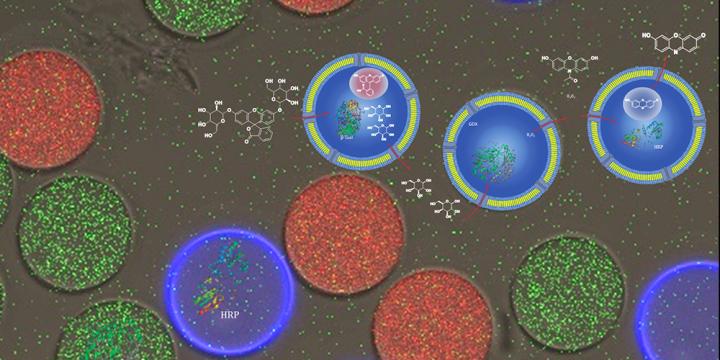
“Here, an elegant strategy for studying such behavior, by controlling segregation/combination of enzymes/metabolites in synthetic cell‐sized compartments, while preserving vital cellular elements is presented. Therefore, compartments shaped into polymer GUVs [Giant Unilamellar Vesicles] are developed, producing via high‐precision double‐emulsion microfluidics that enable: i) tight control over the absolute and relative enzymatic contents inside the GUVs, reaching nearly 100% encapsulation and co‐encapsulation efficiencies, and ii) functional reconstitution of biopores and membrane proteins in the GUVs polymeric membrane, thus supporting in situ reactions.”
“GUVs equipped with biopores/membrane proteins and loaded with one or more enzymes are arranged in a variety of combinations that allow the study of a three‐step cascade in multiple topologies. Due to the spatiotemporal control provided, optimum conditions for decreasing the accumulation of inhibitors are unveiled, and benefited from reactive intermediates to maximize the overall cascade efficiency in compartments.
“The non‐system‐specific feature of the novel strategy makes this system an ideal candidate for the development of new synthetic routes as well as for screening natural and more complex pathways.”
Given the complexity of processes in living cells, it is impossible to determine when specific enzymes are present at what concentrations and what their optimum proportions are relative to one another, note the researchers, who use smaller, synthetic systems as models to study these processes. These synthetic systems simulate the subdivision of living cells into separate compartments.
Now, the team led by Cornelia Palivan, PhD, and Wolfgang Meier, PhD, from the department of chemistry at the University of Basel, has developed a new strategy for producing these synthetic systems. The new method allows the researchers to tweak the size and composition of the different vesicles so that various biochemical reactions can take place inside them without influencing one another—like in the different compartments of a cell.
The researchers used a newly developed microfluidic platform to produce three different types of vesicles with a uniform size but different cargoes: β-galactosidase (red vesicle), glucose oxidase (green vesicle) or horseradish peroxidase (blue). The water-soluble enzymes gradually convert the starting product into the final colored product Resorufin which, like all of the intermediates, enters the surrounding solution via selective channels in the vesicle membranes.
“We were able to show that the new system offers an excellent foundation for studying enzymatic reaction processes,” says Palivan. “These processes can be optimized to boost the production of a desired final product. What’s more, the technology allows us to examine specific mechanisms that play a role in metabolic diseases or that affect the reaction of certain drugs in the body.”