November 15, 2010 (Vol. 30, No. 20)
Novel Dimensions and Capabilities Keep Technology Fresh and in Demand at Labs
Flow cytometry is and always has been a multiparametric technology. Increasingly simultaneous and correlated measurements can be used to provide greater detail about the phenotype of cellular subpopulations. Clinical laboratories now routinely employ 5- or 6-color flow cytometry, while research labs are experimenting with 10- to 20-color capabilities.
Innovations such as image flow cytometry and acoustically focused flow cytometry have added new dimensions and capabilities, and in some cases flow cytometers have begun to replace other instruments like microscopes.
At the same time, in order to reach its full potential, the field of flow cytometry must overcome some significant challenges. Chief among these is the abundance of data generated from increasingly large multiparametric analyses.
At the other end of the spectrum, there are some basic problems that are still being solved, such as how to accurately calculate the sensitivity of the instrument. Potential solutions to a range of problems impeding the efficient use of flow cytometry were presented at the recent “Great Lakes International Imaging and Flow Cytometry Association” conference.
Deconstructing Detection Limits
Many flow cytometer sensitivity measurements do not accurately represent the sensitivity of the instrument, according to Eric Chase, president and CEO of Cytek Development. Typically, detection limits are used to describe instrument sensitivity, equating the noise of the instrument to the mean equivalent fluorochrome (MEFL) value, which is the number of dye molecules the instrument can supposedly detect.
“That number is kind of meaningless,” according to Chase, because the detection limit is not really a function of the performance or optical sensitivity of the cytometer. It’s more a function of the design of the electronics.
“In newer flow cytometers, the way the electronics work, that number should always be zero because the newer digital flow cytometers use area systems,” Chase added. “They give a detection limit of zero.”
That doesn’t mean, however, that the instrument can pick out one or two dye molecules from background noise, which is the kind of information intended to be conveyed by a sensitivity rating.
Chase said that manufacturers continue to use detection limits because the numbers are low and it makes the instrument seem sensitive. A typical detection limit would be 100 dye molecules or less.
Chase’s method is based on values called Q and b that describe flow cytometer performance. Q is related to the ability of the instrument to capture signals, and b is a measurement of the background of the instrument. Although BD Biosciences adopted Chase’s method in its cytometer quality-control package, he says that the Q and b of the instrument do not give information that is directly actionable in the laboratory.
He described how Q and b could be used to calculate a resolution limit, analogous to MEFL, but that describes how many molecules of dye a user needs to be fully resolved from the background noise.
This is one step away from the number of antibody-binding sites on a cell that the instrument can detect, he explained. Users can calculate their own resolution limit based on their instrument’s Q and b values, but more support from instrument and reagent vendors would be necessary for determining the number of antibody sites an instrument could resolve.
Chase foresees a future where resolution limits will be industry standard and every reagent bottle will come labeled with the necessary information for making the calculation. This would represent a major advantage to the user, but first users and vendors alike will have to let go of their attachment to superlow—but not necessarily accurate—detection limits.
The Cellular Social Networker
Single-cell network profiling applications were discussed by Todd Covey, a scientist at Nodality. Nodality developed its platform in response to the lack of technologies that can measure signal-transduction networks in drug development and in clinical medicine.
The technology uses quantitative flow cytometry to measure phosphoproteins in signaling pathways inside an individual cell. A cancer cell, for example, has a pathway signature unique to its genetic and epigenetic changes.
A large proportion of drugs in development are kinase inhibitors, but many fail because the drug is not being correctly used in the patient population with the relevant biology, according to Covey.
The Nodality approach can be used in the drug-development field to identify what pathways a drug is targeting, or in a clinical application to define a patient population. Later, the two approaches merge as patient and drug are matched based on single-cell network profiles, and biology of tumor is matched to mechanism of drug.
“Each cell is its own separate measurement,” said Covey. “We’re able to look at signaling in a variety of different cell populations at once. We’re able to stimulate multiple pathways in different cell populations and see the activity of a certain drug in different cell pathways.”
In a case study of an 83-year-old man with chronic myelogenous leukemia being treated with Dacogen (decitabine), Covey tested multiple drugs including a number of types of targeted kinase inhibitors in various combinations and was able to observe the effects on apoptosis and cytostasis within different tumor populations. One of his findings was that decitabine was active against one cell type of the tumor, but not potent against another cell type.
“Being able to pull apart heterogeneous cell types and see different drug effects is powerful,” Covey remarked, “especially as you monitor the patients along their disease course.”

Functional tumor characterization of blood cells in a patient with chronic myelomonocytic leukemia (CMML): For each cell subset, signaling activity (boxed proteins) is measured in the presence and absence of drug treatment (yellow boxes) across multiple pathways representing diverse biology. During disease progression, changes in the signaling profile and therapeutic sensitivity/resistance can be monitored. [Nodality]
Toxic Assets
In his presentation, Joseph Tario Jr., predoctoral research affiliate at the Roswell Park Cancer Institute, explored a new way of exploiting the power of flow cytometry in a practical, clinical research setting. Tario and his colleagues developed an assay for assessing cytotoxicity by flow cytometry, using components easily accessible to most laboratories.
Cytotoxicity assays are useful in cancer research because they can monitor whether a drug or therapeutic intervention can render the immune system capable of killing tumor cells. This contrasts to other readouts such as the measurement of immune cell numbers or their phenotypic activation status. While such investigations are meaningful, they provide little information as to whether the cells are capable of eliminating disease.
“Cytotoxicity is an important metric in determining the success of an immune response. Conversely, the absence of cytotoxicity when it is expected to occur may indicate that there’s a suppressive environment that is being imparted upon the cell system,” Tario pointed out.
Restoring the immune system’s ability to kill cancer cells is an important strategy in developing anticancer therapies. Flow cytometric methods of measuring cytotoxicity have been shown to compare favorably to the current prevailing methodology, the chromium release assay, which requires the use of dangerous radioactive reagents, often exhibits high backgrounds, and is entirely uniparametric.
In the flow cytometric assay, effector and target cell populations were discriminated from one another with two different lipophilic dyes and viability was assessed with a DNA intercalating reagent. Samples were normalized to controls via the use of fluorescent enumeration beads, and data was acquired on a 14-color LSRII flow cytometer from BD.
The assay gives an accurate readout of cytotoxicity, but its true power comes from the ability to combine cytotoxicity with additional simultaneous and correlated measurements such as antigen-expression profiles and cytokine production in order to more fully elucidate how an intervention affects immune cells.
“Our work on the improved cytotoxicity assay is a step in a process that represents a paradigm shift toward the increasing role of flow cytometry in the laboratory, Tario concluded. “Due to its multiparametric nature, flow cytometry yields data that is inherently more useful than uniparametric technologies such as chromium release. Furthermore, flow cytometry is logistically more feasible than many of the technologies it is capable of replacing.”
Which Way to the Future?
Trends in flow cytometry favor smaller, more powerful lasers, instruments capable of larger numbers of simultaneous measurements, and increasingly advanced software to handle the resulting data avalanche. In the category of instruments for research analysis, manufacturers are pushing the boundaries of analysis in two directions. Using greater numbers of lasers and dyes allows researchers to carry out experiments that measure many parameters simultaneously. At the same time, smaller, simpler flow cytometers are being developed that can be taken into the field and into developing countries to do CD4 counts for HIV testing.
These trends could take flow cytometry in a number of different directions, according to Bill Rhodes, president of BD Biosciences.
“In terms of cutting edge, what you’re looking at is the ability to sort a wide variety of sizes of cells in ways that keep them viable, and to sort them using multiple parameters,” Rhodes said. “A lot of cell sorting now is being looked at for the purposes of investigating disease and perhaps, using induced pluripotent stem cells, dendritic cells, or other types of regulatory cells for the purposes of therapy.”
For example, in the future, flow cytometry could be used to purify stem cells for bone marrow transplantation. Advanced cell sorting could help find and eliminate extremely rare cells and improve the odds of success by using a more highly purified donor sample.
“There are probably dozens, if not more, trials now around the world where flow cytometry is being used in conjunction with bulk separation to get to that high level of purity,” Rhodes added.
For researchers, Rhodes predicted that, in addition to better instruments for power users, there will also be flow cytometry options for researchers who may not be experts at interpreting fluorescence spectra or calibrating optics but who want to use flow cytometry analysis in their work, for example in marine biology and environmental science. Those systems would have one or two lasers, using three or four colors at a time, but would be equipped with advanced software in their user interface.
Broad Stream, Tight Spot
Giacomo Vacca, Ph.D., R&D program manager and research fellow at Abbott Laboratories drew interest with his presentation on laser rastering in flow cytometry. Three features distinguish laser rastering flow cytometry from conventional flow cytometry. First, instead of narrowing the stream of fluid to a single line of cells, the laser-rastering system uses a wide stream with many cells coming through at the same time to increase throughput.
Second, instead of a broad, elongated spot, it uses a tight laser spot in both dimensions. And lastly, instead of a fixed laser beam that waits for cells to flow past, the laser beam is scanned, or rastered, across the core stream as the cells go by, and interacts with each cell multiple times.
Dr. Vacca and his colleagues have been building and perfecting laser rastering flow cytometry at Abbott Hematology for several years with the goal of creating a better hematology analyzer. “We can measure the same things you can in a flow cytometer or hematology analyzer, but at a much greater rate,” he said.
The large amount of complex data does, however, require some “heavy-duty signal processing.” The system can process more than 300,000 cells per second, claims Dr. Vacca, whereas a high-end hematology analyzer can handle only about 10,000 to 20,000 cells per second. One advantage of this higher rate might be to scan for rare events.
One common problem in using a flow cytometer to analyze blood cells is called the coincidence problem. This is when two cells are in the beam at the same time and cannot be resolved. Surprisingly, Dr. Vacca’s laser-rastered system does not have a greater degree of coincidence than a conventional flow cytometer because the wider stream is compensated for by the tighter laser spot.
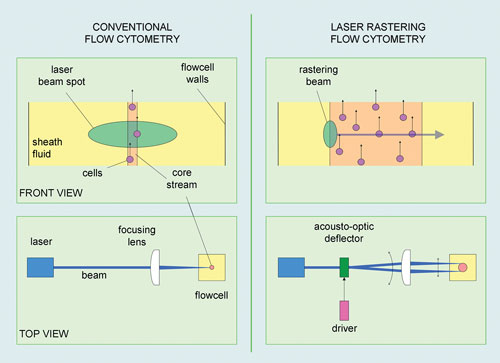
Over the last several years, researchers at Abbott Laboratories have been building and perfecting laser rastering flow cytometry. Three features distinguish laser rastering from conventional flow cytometry: instead of a single line of cells, a wide stream is used; instead of a broad elongated spot, a tighter laser spot in both dimensions is utilized; and, finally, the laser beam is scanned or rastered across the core stream as the cells go by.
Tips on how to maximize cytometry operations