February 15, 2017 (Vol. 37, No. 4)
Investigating Aberrant Chemotactic Behavior in Fibroblasts from Patients with Lung Disease
Chemotaxis is a mechanism that provides directional cellular movement in response to alterations in the chemical composition of the immediate environment. The mechanism, in its simplest form, governs a wide range of biological processes: from targeting the movement of neutrophils toward a site of infection to providing cancer cells with a means of entering circulation during metastasis. It is also well known that chemotactic cell migration is a significant factor in the coordination of many physiological processes, such as wound healing and embryonic development.
Due to the far-reaching implications of chemotactic activity in clinical research, it will come as no surprise that the underlying mechanisms that govern the process have long been investigated. There are several well-established techniques, but recent technological advances enable scientists to now view chemotaxis in much finer detail.
Overcoming Hurdles in Current Technology
Conventionally, researchers have employed long-established techniques for the investigation of cell migration, such as transwell-based migration assays. In these assays, cells of interest are incubated in a well that has a built-in, cell-permeable membrane, creating a two-chamber environment. Cell counts are conducted to establish the number of cells that have migrated across the membrane into the second chamber, which contains a chemical attractant. Although migration assays have their strengths in identifying differences in migratory action between different groups of cells, the data cannot provide insights into the particular paths taken by individual cells. In addition, the molecular gradients themselves are undefined and unstable.
Owing to the significant role played by chemotaxis in biology, novel methods are under development for an enhanced characterization of the process. Researchers are combining microfluidics, live-cell imaging, and cell tracking to enable them to more precisely monitor phenomena such as cell velocity. This means a distinction can be made between chemotaxis and chemokinesis, and researchers are able to better control specific chemical gradients so as to understand cellular behavior under different conditions.
Microfluidic assays, unlike conventional migration techniques, can provide cells with a continuous flow of media containing chemoattractant, which can be used to form a gradient that more closely matches that found in nature. The coupling of this technique with live-cell imaging means chemotactic cells can be distinguished from cells passively migrating, and individual cells can be tracked to provide researchers with a wealth of information regarding their response to particular chemoattractants.
A Closer Look at Chemotaxis
In order to assess the efficiency of this approach as an update to conventional migration assays, researchers investigated aberrant chemotactic behavior in fibroblasts from patients with inflammatory lung disease. It is thought that the migratory behavior of these cells in patients suffering from pulmonary diseases may be significant for disease progression. Previous research has shown that fibroblasts taken from fibrotic lung tissue elicit a chemokinetic response when exposed to platelet-derived growth factor (PDGF), unlike fibroblasts taken from healthy patients.
Although the research highlighted an increased chemokinetic response, the question remains as to whether the chemotactic behavior of the cells is enhanced in the presence of PDGF. Cells were therefore taken from patients suffering from asthma, cystic fibrosis and chronic obstructive pulmonary disease (COPD). Live-cell imaging with a microfluidic assay, in this instance using the Lonza CytoSMART™ System in combination with the CellDirector® 2D by Gradientech, was used to quantify their response to a gradient of PDGF.
Both normal and diseased human lung fibroblasts were cultured to 80% confluence, and the migration experiments were conducted in triplicates for the different disease groups, with positive and negative controls.
On the first day of experimentation, cell suspension was pipetted into the microfluidic kit and incubated overnight to allow for cell adhesion. Once cells had adhered, syringes were connected to the inlet connectors of the chamber. Both syringes contained serum-free medium, but only one contained PDGF. A concentration gradient was achieved via connecting the syringes to a syringe pump, which allows the syringe contents to flow through the assay past the adhered cells. The chamber was then placed in the image acquisition holder, and the fibroblast responses in both gradient and control channels were monitored in real time for a duration of five hours, producing a total of 40 images per experiment.
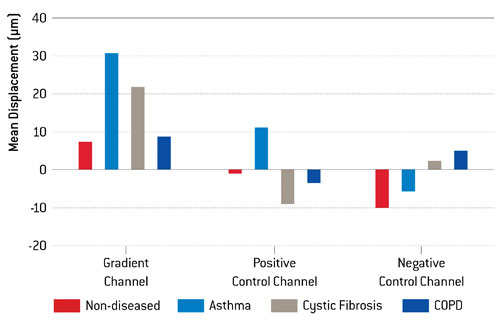
Figure 1. Center of mass (COM) displacement of fibroblasts from different disease groups (as well as healthy cells) as an average, taken from triplicate experiments.
The combination of the chemotaxis assay, live-cell imaging, and tracking software provided researchers with data on factors such as center of mass displacement (COM)—an indication of the mean displacement of cells that can be used as a measurement of chemotactic behavior. This is a far more accurate and precise way of measuring chemotaxis when compared to well-based migration assays, and assessing COM can help distinguish true chemotactic cells in a sample from cells moving nondirectedly. This data was ascertained via the analysis of the image sequence by the Tracking Tool™ PRO tracking software, following the initial experiment. As shown in Figure 1, fibroblasts obtained from patients suffering from asthma and cystic fibrosis were more responsive to the PDGF than those from COPD patients—although healthy and COPD fibroblasts did respond to a smaller degree.
In addition, advanced techniques like this can provide researchers with detailed trajectory plots (known as polar plots), where the path of individual cells is recorded and displayed, showing the direction of the chemoattractant gradient. Interestingly, the polar plots and their corresponding statistical data generated by region-specific image analysis seemed to show dose-dependent chemotactic behavior in asthma DHLF, which (as shown in Figure 2) is far more apparent in the lower PDGF concentration interval (between 0 and 12.5 ng/mL of PDGF).
The notion of a dose-response relationship between PDGF concentration and chemotactic behavior could provide researchers with an insight into how cell mobility can be manipulated by altering concentrations of a given chemoattractant. This may offer a potential avenue for the development of treatments for patients suffering from diseases such as asthma, as it may help scientists to better understand the conditions in which the disease is able to progress. The relationship was identified via the software-based analysis of data obtained from the microfluidic and live-imaging techniques outlined. This includes the analysis of polar plots and differences in cell velocity, COM, forward migration index, and cell directness. This represents a vast improvement in terms of the depth of data that can be obtained to explain this phenomenon, particularly when compared to conventional migration assays.
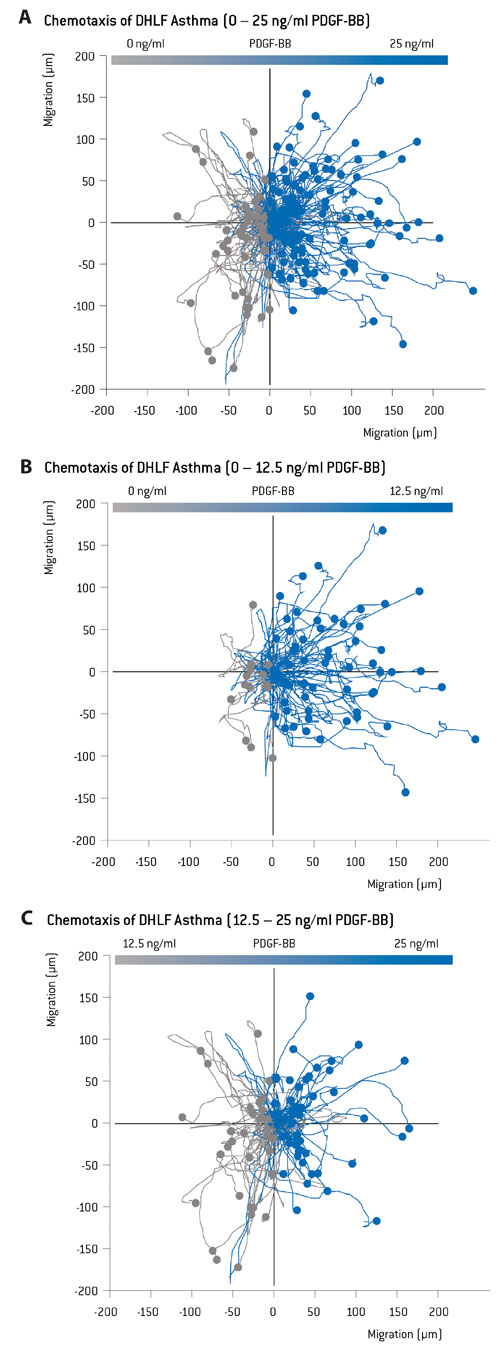
Figure 2. Polar plots showing chemotaxis of fibroblasts obtained from asthma patients (DHLF Asthma). The data show that there is a greater proportion of directional movement by the fibroblasts that are exposed to a concentration gradient of 0–12.5 ng/mL, compared to the channel area with higher concentrations.
Conclusion
Chemotaxis is a major feature in a number of disease processes—utilized by wound healing, cancer, and immune cells alike. This means experimental techniques that allow for the precise investigation of the finer details of the process are required to further enhance our understanding. Conventional assays offer little data in terms of the directionality of individual cells, and are unable to accurately mimic conditions found in nature. Such drawbacks mean that the adoption of novel techniques like the ones outlined in this article are likely to provide researchers with the tools they need to expand their knowledge of many cellular processes.
Claudia Schwartz, Ph.D., is product marketing manager at Lonza Bioscience. Contact [email protected] for more information.