December 1, 2016 (Vol. 36, No. 21)
Finding a Suitable Target Antigen Is a Key Difficulty that Is Being Addressed
The road to developing effective cancer immunotherapies using chimeric antigen receptor (CAR) T cells has been a long and sometimes rocky one.
However, in recent years this therapy has shown remarkable success in patients with hematologic or “liquid” cancers.
These successes have predominantly involved using CAR T cells, targeting the CD19 receptor in patients with leukemia. For example, in April this year a Phase I/II trial in the U.S. reported that 27 of 29 acute lymphoblastic leukemia (ALL) patients who had been resistant to other forms of treatment had gone into apparent remission after receiving genetically engineered CD19 CAR T cells. This type of therapy is also being tested in patients with lymphoma and myeloma.
After observing the potential of this form of treatment for seriously ill patients with liquid tumors, researchers and clinicians are keen to apply this technology to more common solid tumors such as ovarian and pancreatic cancer.
Although a number of recent trials have targeted antigens found on solid tumors, such as HER2, clinical results have been disappointing. In two of the most promising trials reported to-date, only 3 of 11 neuroblastoma patients treated with GD2 CAR T cells went into remission and only 4 of 17 sarcoma patients treated with HER2 CAR T cells went on to develop stable disease.
The pitfalls of using CAR T-cell therapy to treat solid tumors and how to address them was a key theme discussed by a number of researchers in November at CHI on Novel Immunotherapy Strategies, in Lisbon.
Solid versus Liquid Tumor Targeting
It was widely agreed among the CAR T-cell therapy experts that a key difficulty with targeting solid tumors is finding a suitable target antigen. Renier Brentjens, M.D., Ph.D., director, cellular therapeutics center, Memorial Sloan Kettering Cancer Center, has been involved in the race to develop effective CAR T-cell therapies for many years.
“There is a reason why several groups, including our own, initially tried this technology targeting CD19: because it’s a relatively safe target and it is pretty ubiquitously expressed by the tumor cells,” he explained. “In solid tumors there is a significant amount of heterogeneity and so even if you find a reasonably ideal target antigen that isn’t expressed on normal tissues, the likelihood that all of the tumor cells would express that target antigen is pretty small.
“So the risk is that you can kill off some of the tumor, but the rest of the tumor simply escapes detection.”
Another issue with targeting non-hematologic tumors is that achieving a good level of tumor penetrance is harder, say the researchers. While the liquid nature of cancers such as leukemia and their presence in the blood makes them easy to access, this is not the case for solid tumors. The large volume of some solid tumors can also be a problem, as it’s difficult for the T cells to eradicate the whole tumor.
“Solid tumors create a local microenvironment that is highly immunosuppressive and overcoming that suppressive state is a challenge,” said Andrew Kaiser, Ph.D., R&D manager and senior group leader, Miltenyi Biotec.
Dr. Kaiser’s company released a closed, single-use tubing set this year that allows users to reproducibly select, modify, and expand T cells extracted from a patient’s blood to be used as a therapy within two weeks.
This tumor microenvironment means that even if the researchers can find a good target antigen and generate CAR T cells that can find the tumor, the T cells may end up simply being suppressed and de-activated on arrival.
Soldano Ferrone, M.D., Ph.D., Massachusetts General Hospital and Harvard Medical School, is an expert on the escape mechanisms utilized by tumor cells to avoid immune recognition. Highlighting one aspect of the solid tumor environment that can disrupt treatment, he noted that “There is much more hypoxia, which we have learned can cause several problems. These range from increased frequency of cancer initiating cells to reduced susceptibility of the tumor cells to the lytic activity of the CAR T cells.”
Dr. Ferrone added that another source of environmental problems when treating solid tumors is exosomes (cell-derived vesicles) in the blood.
“There is more and more evidence that the exosomes will inhibit the activity of not only CAR T cells but also other types of effector cells,” he said.
Sustainability of the CAR T cells once infused into the patient is something that seems to be more of an issue with solid than with liquid tumors. Magnus Essand, Ph.D., Uppsala University, an expert on cancer immunotherapy, believes that the regular interaction that B cells have with T cells in the blood means that CAR T cells targeting B-cell malignancies may stay in the patient’s system for much longer than those targeting solid tumors.
He explained that the choice of T-cell type used to create the CAR T-cell infusion and the way the cells are cultured can also have an impact on how effective they are at treating the cancer.
“I think we can improve the protocols of how we expand the T cells, because they are rather fatigued by the time they are given back to the patients by the way that we culture them today,” he said.
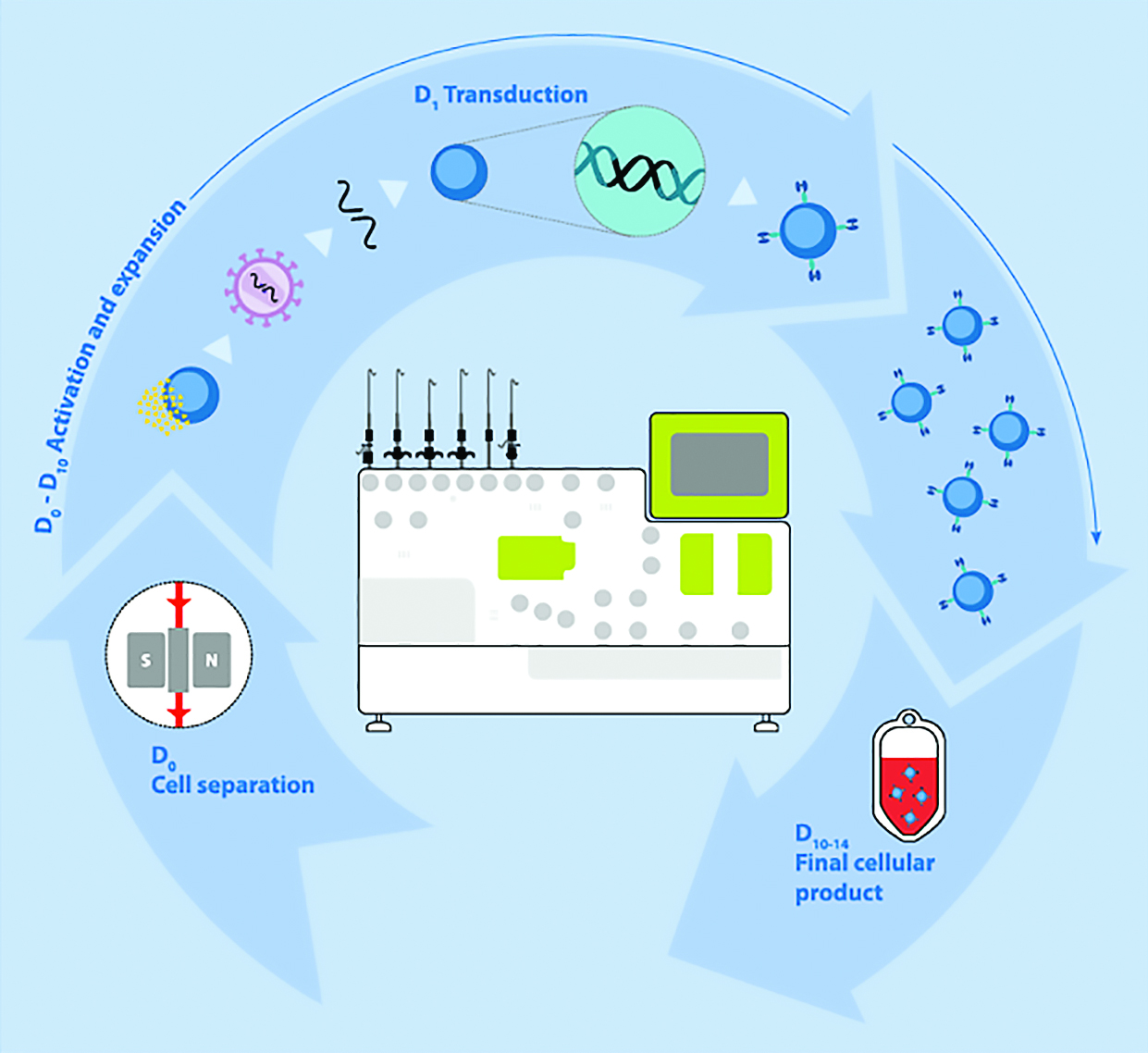
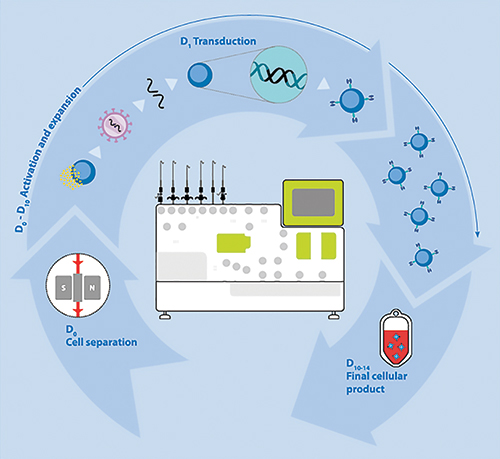
The workflow for automated manufacturing of gene-modified T cells using Miltenyi Biotec’s Prodigy platform.
Driving Therapy Forward
Despite the evident complexity of targeting solid tumors using CAR T cells, there is still optimism from researchers that effective therapies are on the horizon.
Michael Hudecek, M.D., Ph.D., Würzburg University, and his team have developed a CAR T cell specific for the antigen ROR1 that is expressed on epithelial cancers. He explained the importance of optimizing the design of the CAR.
“We have done a lot of optimization on the design of the ROR1 CAR. We’ve shown the particular epitope on the ROR1 molecule that is targeted is important and that the CAR spacer design has to be adjusted so that an effective immune synapse between T cells and tumor cells can be formed,” he told GEN.
“We are also doing more comprehensive engineering where we are not only inserting the CAR gene, but we are also doing genome editing to knock-out certain proteins or functions in T-cells so they are more resistant to immunosuppression, for example.”
Testing of the ROR1 CAR T cells is still at an early stage, but Dr. Hudecek says that the work they have done so far, which has included using realistic 3D tumor models created by his colleagues in the tissue engineering department at Würzburg University, has been positive. The first clinical trials of ROR1 CAR T cells in humans are about to begin in Seattle, he added.
Dr. Hudecek points out that using CAR T-cell therapy to treat solid tumors is a treatment that is still in its infancy. “I think it’s too early to judge whether CAR T cells have or have no clinical efficacy because there have been just a very limited number of studies,” he emphasized.
In addition to good CAR design, a number of factors need to be overcome to create an effective therapy for solid tumors. Surface antigens need to be found that can be targeted without adversely affecting healthy tissues, the immunosuppressive microenvironment needs to be overcome, and the CAR T cells need to be able to move easily through the body and into the tumor to kill the malignant cells.
“Such improvements can be achieved by further manipulating the T-cells genetically and/or using specific culture conditions and by manipulating the host (for instance removing the defective immune cells by lymphodepletion prior to giving “newly educated“ T-cells),” commented Dr. Kaiser.
Solid tumors are often formed of more than one cell type and it is important to recognize that even with a good target antigen, it is likely that some of the tumor cells will not express it and will therefore evade detection by the CAR T cells.
“You need to activate bystander immunity…to kill off the tumor cells that don’t express the antigen for the CAR molecule, because otherwise you will develop clones with antigen negative tumors,” explained Dr. Essand.
“You need to have the CAR T cells express a cytokine, for example, that can attract other effector immune cells to come there and help them…The T cells will not be able to do the job all by themselves, they will need help,” he added.
To achieve effective remission, it is also important to target cancer initiating and sustaining cells, because if left behind they will be a source of recurrence and metastatic spread, specialists believe.
“There is clinical evidence that targeting such cells can be effective (e.g., rituximab in melanoma),” said Dr. Kaiser. “Cancer-sustaining cells are thought to be present in all metastases and in many cancer types and effectively eliminating such cells could make a difference in many indications.”
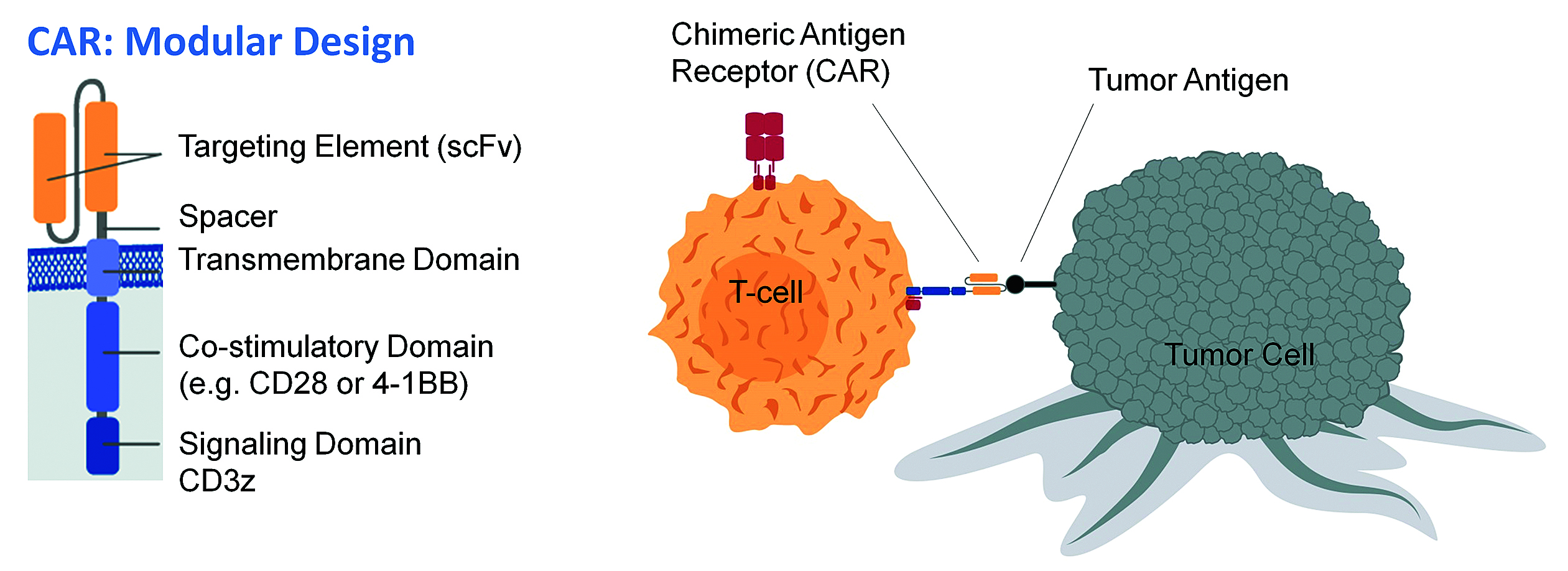
CARs are synthetic receptors with a modular design. The extracellular domain allows efficient and HLA-independent binding to the surface molecule on tumor cells. The VL and VH segments (scFv) of the monoclonal antibody are used as targeting elements. This scFv is linked to a spacer domain. In contrast to TCR-engineered T-cell therapy, this construct allows an interaction between the T cell and the tumor cell that is independent of HLA. By linking the extracellular domain to a transmembrane domain of human CD28 or the cytoplasmic domain of human 4-1BB, the receptor is anchored within the cell membrane of the T cell. For activation of the T cell, a signaling domain, mostly CD3 zeta, is added. Dependent on the generation of CAR, second- and third-generation CARs also contain one or several co-stimulatory domains that support signaling. [Würzburg University, hudeceklab.org]
Safety First
In recent months, CAR T-cell therapy has hit the headlines because of a number of patient deaths that occurred in trials of CAR T-cell therapy being conducted by Juno Therapeutics and Ziopharm.
Minimizing toxicity and maximizing patient safety is a key concern for researchers developing CAR T-cell therapy for solid tumors.
“We have to realize that what we inject is very active, which is useful because it can eliminate tumors, but by the same token it’s something that if it hits the wrong tissue it can cause a lot of problems,” said Dr. Ferrone. “We have to do all our homework to be sure that we don’t have unexpected surprises.”
Attilio Bondanza, M.D., Ph.D., San Raffaele University Hospital and Scientific Institute, specializes in cancer immunotherapy and has been involved in developing so-called suicide genes to help switch off engineered T cells. He and his colleagues have also modeled cytokine release syndrome (CRS), the extreme and sometimes catastrophic immune reaction seen in some patients treated with CAR T-cell therapy, to try and predict who is most likely to experience it.
“There are some patients who are more prone to develop CRS, possibly because of polymorphisms in immune-related genes, than some others. There are also some clinical variables, such as tumor burden, type of disease, that have a high positive predictive value,” explained Dr. Bondanza.
He added that while suicide genes are an important safety feature for this type of therapy, they should only be triggered as a last resort, or after the T-cells have had time to take effect.
Most CAR T-cell therapy is introduced into the patient’s system using a viral vector. However, Dr. Hudecek and his team are using nonviral gene transfer in their research.
“I think it’s part of the safety strategy to use a gene transfer strategy that has the best safety characteristics and this is currently the nonviral strategy,” he said.
Dr. Bondanza explained that an important result of successful CAR T-cell therapy for solid tumors would be reduced costs, as this type of treatment is currently very expensive to produce.
“Hematologic cancers are rare tumors and treated in very specialized centers, so if you want to treat much more common solid tumors… sooner or later you have to democratize the generation of these T cells, by reducing costs and making them much more available,” he noted.
Drs. Kaiser and Bondanza are both involved in a European project called CARAT that aims to tackle all aspects of CAR T-cell therapy to advance the field and widely disseminate improvements. The project involves a consortium of renowned experts in Germany, France, Italy, and the U.K.
“Hopefully these efforts will advance such promising treatments so that they are accessible to a much larger patient population,” said Dr. Kaiser.
“It’s going to be hard to predict what form this technology will be in in 10 years, but I feel confident that it’s a technology that will have application to not just relatively rare and select cancers but to a broader pool of both liquid as well as solid tumors,” commented Dr. Brentjens. “It’s just a matter of giving us time and being creative in ways to generate better T cells that can do the job even when that job is a bit more difficult.”
High-Throughput Flow Speeds Profiling
Personalized immunotherapies are based on assessments of immune status in patients by profiling cell function and phenotype to yield a “signature” of the interaction between immune cells and tumor cells. While these treatments hold great promise, there is a need for faster, better ways to assess immune status and to visualize insights into large combinatorial datasets in order to guide immunotherapy.
Multiparameter flow cytometry has shown great utility in immunology research to profile immune cells for function and phenotype. However, traditional flow cytometry suffers from lack of high sample throughput; it requires too many cells (which are often difficult to procure) driving up the assay and reagent cost, requires a dedicated expert to operate the system, and lacks the software tools to correlate the high-content data inherent in large-scale immune profiling workflows, according to Joe Zock, senior director of product management at IntelliCyt.
“The company’s iQue® Screener platform has been widely adopted by immuno-oncology centers in pharmaceutical, biotechnology, and academic medical centers due to its ability to rapidly and easily perform immune profiling,” he says. “Plate-centric in design and automated in function, microplates can be sampled and analyzed in minutes (96-well plates in under 5 minutes, 384-well plates in under 20 minutes) using small assay volumes to save precious cells.”
The platform identifies various subpopulations, assesses functions (e.g., viability, proliferation, apoptosis, necrosis, cell cycle, etc.), and captures cytokine profiles from each small sample. The ForeCyt® Software analyzes collected data and facilitates “mining” the resulting high-content picture of biological responses for user-defined signatures that identify the wells of interest as well as generating dose response curves.
“Resulting insights into the complex biology of the immune response can now be routinely done to discover correlates of clinical response,” notes Zock.
An example from adoptive cell therapy workflow is to rapidly assess the cancer killing potential of the multiple CAR T-cell preparations by mixing them with a patient’s own cancer cells or suitable surrogates. CAR T-cell activation, cell health, and killing can all be measured simultaneously using extremely small quantities of cells, allowing researchers to choose the best clone to expand for therapeutic return to the patient.
“These cell mediated cytotoxicity assays are being used for functional assays of checkpoint inhibitors as well as assessing synergy in combinatorial therapy studies,” adds Zock.

IntelliCyt maintains that its iQue Screener platform enhances screening workflow from sample preparation through results.