April 15, 2018 (Vol. 38, No. 8)
With Multiplexing, Nanodelivery, and Other Advances, CRISPR Reality Outstrips CRISPR Fiction
CRISPR truth is stranger than CRISPR fiction, where the revolutionary gene-editing technology is being exploited as a fairly conventional plot device. In an X Files season finale, aliens weaponize CRISPR to attack the human immune system. In Change Agent, a science fiction novel that has been optioned by Netflix, a criminal syndicate uses CRISPR to develop a range of illicit genetic services. And in C.R.I.S.P.R., a procedural thriller that Jennifer Lopez hopes to develop for NBC, a CDC scientist and an FBI agent flirt with romance while thwarting the schemes of a mad scientist.
If CRISPR fiction seems predictable, it’s not the fault of CRISPR, but fiction, which is necessarily limited (as one literary critic suggested) on account of its origin—our shared desire for meaning. Caring nothing for human conventions, CRISPR retains the ability to surprise. After all, CRISPR (which stands for clustered, regularly interspaced, short palindromic repeats) evolved to protect bacteria from bacteriophages long before we decided to adapt this host-defense system for our own uses.
If we are to find endlessly diverting CRISPR programming, we need to pay attention to real CRISPR stories in basic research, therapeutics development, and agricultural technology. Fortunately, tuning into CRISPR can be as easy as attending a conference or reading GEN. Spoiler alert: In this article, GEN summarizes some of the most interesting stories from Precision CRISPR, which recently held its fourth annual precision CRISPR congress. This event showcased multiplexing platforms, engineered nanocarriers, next-generation chimeric antigen receptor (CAR) T-cell therapies, and advanced plant breeding technologies.
Alternative Nucleases
Typically, a protein of the CRISPR-associated (Cas) family of nucleases complexes with a piece of RNA, which guides the nuclease to a DNA sequence that has a complementary base sequence, where the nuclease cuts the genome. (Alternatively, a catalytically dead version of the nuclease may bind to the DNA, interfering with expression and modulating the epigenome.) The most widely used Cas nuclease, SpCas9, derived from Streptococcus pyogenes, targets a 20-nucleotide DNA sequence that is immediately followed by an NGG (aNy base-Guanine-Guanine) sequence, that is, a PAM (protospacer adjacent motif).
Naturally occurring Cas9 proteins sometimes lose the plot themselves, producing off-target effects or demonstrating limited target range. Consequently, researchers hope to develop improved versions of Cas9. “We wanted to engineer even safer and more targetable tools for research and therapeutic applications,” said Benjamin Kleinstiver, Ph.D., one of the presenters at the Precision CRISPR event.
Dr. Kleinstiver, an instructor of pathology at Massachusetts General Hospital and Harvard Medical School, works in the laboratory of J. Keith Joung, M.D., Ph.D., which has been working to optimize SpCas9 and other Cas proteins such as SaCas9 (Staphylococcus aureus Cas9) and Cas12a (formerly Cpf1, or CRISPR from Prevotella and Francisella 1). These Cas9 nucleases are proving especially useful for gene editing in human cells.
Like Cas9, CRISPR-Cas12a nucleases also are targeted to specific DNA sequences via a single guide RNA (sgRNA). The Cas12a-sgRNA complex scans the genome for the presence of a suitable PAM, which in this case is TTTV (Thymine-Thymine-Thymine-not thymine). Dr. Kleinstiver noted, “Cas12a enzymes have some unique properties, including the ability to recognize a different PAM motif, expanding the number of editable sites in the genome.”
Another advantage of Cas12a is superior multiplexing capability. Unlike SpCas9, which cuts only DNA, Cas12a has dual RNase and DNase activity.
“We and others have shown that Cas12a can process individual sgRNAs out of a single long poly-sgRNA transcript,” asserted Dr. Kleinstiver. “My colleague, Esther Tak, Ph.D., combined this property with some additional engineering to modulate the expression of multiple endogenous genes in human cells. This multiplex capability can be used to study the effect of combinatorial gene expression changes.”
Nanoparticle Carriers
Ultimately, for CRISPR to live up to its well-deserved hype, scientists must overcome its in vivo delivery limitations. The CRISPR system requires at least two critical components, the Cas nuclease and the sgRNA. Cas can be delivered directly, as a protein, or in the form of DNA or RNA encoding the protein. In vivo vehicles for transporting these payloads include viral vectors and nanoparticles.
“Several viruses, including lentivirus, adenovirus, and adeno-associated virus (AAV), have been investigated,” noted Daniel G. Anderson, Ph.D., a professor of applied biology, an associate professor of chemical engineering, a member of the Institute of Medical Engineering and Science, and a member of the David H. Koch Institute for Integrative Cancer at the Massachusetts Institute of Technology. “AAV is currently one of the most promising systems.”
“Because of a number of challenges with AAV, scientists are also investigating nonviral delivery systems,” Dr. Anderson continued. “[These systems employ] polymeric or lipid-based nanocarriers that can work without or in combination with viral vectors.”
Nanoparticles have many advantages, including a high loading capacity for nucleic acids, making them suitable for gene delivery. Further, they can be modified to alter the payload’s biodistribution and pharmacokinetics through active targeting and formulation.
Dr. Anderson suggested another intriguing strategy: nanoparticle-based mRNA delivery of CRISPR-Cas components. He advised, “Given concerns over the potential for genomic integration of DNA and potential for genomic rearrangement with long-term Cas9 expression, we believe transient expression of Cas9 through delivery of mRNA could provide a safe and effective approach.”
Whatever methodology is used to introduce the components of a CRISPR system, Dr. Anderson indicated, significant delivery hurdles will be encountered; however, these hurdles can be cleared. “At the end of the day,” he insisted, “utilizing a fully synthetic system, such as nanoparticles, may allow greater customization and could ultimately be the most safe and efficacious way to deliver genome editing therapies.”
CAR T-Cell Therapies
CAR T-cell therapy harvests human T cells and genetically engineers them to attack cancer when infused back into a patient. While the recent approvals of several CAR T-cell therapies have raised excitement and hope, challenges remain.
“CAR T-cell therapies have had unprecedented effects against several human hematological malignancies,” said Devon J. Shedlock, Ph.D., vice president, preclinical development, Poseida Therapeutics. “But these therapies are often not durable and have limited efficacy against solid tumors. Furthermore, autologous therapies are costly and laborious to produce.”
To meet these challenges, Poseida is using its proprietary gene engineering tools to enhance the efficacy of autologous CAR T-cell therapies. The company is also developing technology to produce allogeneic “universal donor” CAR T cells.
“We’ve developed a suite of next-generation tools to overcome many limitations of first-generation CAR T-cell therapies,” asserted Dr. Shedlock. “Our Cas-CLOVER™ hybrid gene editing system fuses a functionally inactive Cas9 to the site-specific nuclease, Clo51. Cas-CLOVER is targeted using a set of two distinct gRNAs, operating like CRISPR-Cas9, but our system has the exquisite specificity of type IIS nucleases and causes no or very few off-target mutations. We can use this high-fidelity system to safely develop off-the-shelf CAR T-cell products.”
While currently approved CAR T-cell therapies utilize viral delivery systems, the field is moving away from use of virus, projected Eric Ostertag, M.D., Ph.D., Poseida’s CEO. “We developed our nonviral piggyBac™ DNA Modification System for efficient gene delivery,” he stated. “It allows for longer lasting and more stable transgene expression. Because it can carry 30 times more cargo, we have been able to introduce CAR along with additional genes including safety switches.”
The company is developing CAR T-cell immunotherapies for multiple myeloma, prostate cancer, and other cancers, as well as gene therapies for orphan diseases. Poseida’s lead product, P-BCMA-101, is being developed to treat multiple myeloma. This CAR T-cell therapy is currently in Phase I clinical testing.
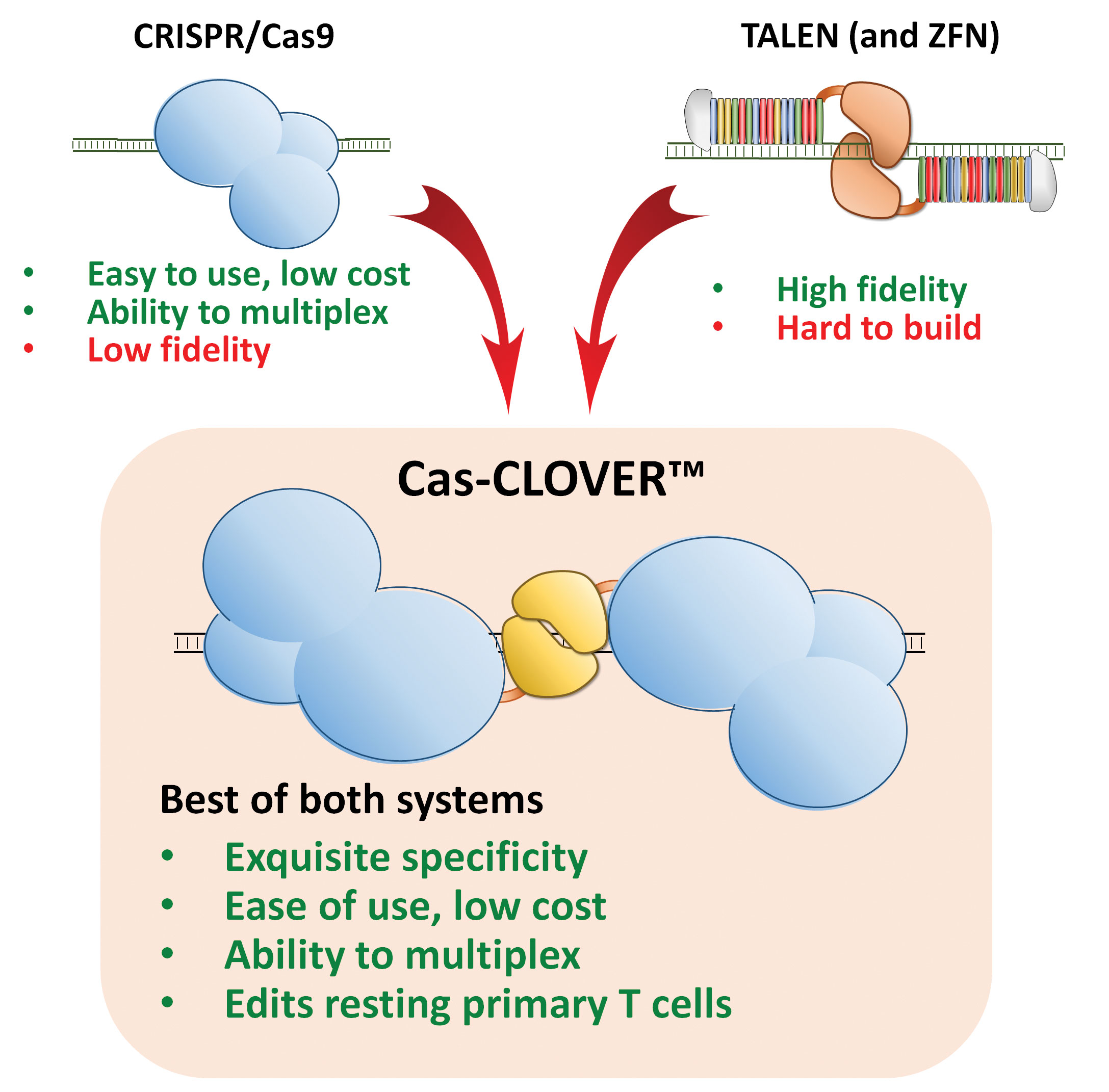
Researchers at Poseida Therapeutics are using Cas-CLOVER™ site-specific nucleases to genetically engineer autologous and allogeneic CAR T-cell therapies targeting hematological cancers and solid tumors. Cas-CLOVER is targeted using a set of two distinct guide RNAs, operating like CRISPR-Cas9, but demonstrating the specificity of type IIS nucleases, causing no or very few off-target mutations.
From Farm to Fork
At the Precision CRISPR event, most of the applications that were discussed focused on advancing therapeutics to the clinic. One presentation, however, was devoted to the acceleration of plant breeding. In this presentation, Greg Gocal, executive vice president and chief science officer, Cibus, described a process called the Rapid Trait Development System (RTDS).
Cibus describes RTDS as a nontransgenic breeding technology that can produce “precise and predictable results with beneficial traits that are indistinguishable from those developed through traditional plant breeding, but with faster results.”
Agricultural CRISPR applications also interest Rodolphe Barrangou, Ph.D., associate professor, Department of Food, Bioprocessing, and Nutrition Sciences, North Carolina University. “CRISPR-Cas technologies excel as tool kits for the food industry,” says Dr. Barrangou, who is also editor-in-chief of the newly launched The CRISPR Journal. He will receive the 2018 National Academy of Sciences prize in Food and Agriculture Sciences for his discovery of the genetic mechanisms and proteins driving CRISPR-Cas systems.
Dr. Barrangou notes that CRISPR-Cas9 applications in food technology range from high-resolution typing of pathogens, vaccination of starter cultures against phages, and the creation of antibiotics that selectively modulate desirable properties in bacterial populations. “The technology is useful not only for enhancing the outcomes of beneficial microorganisms (such as the probiotics and starter cultures used to produce cheeses and yogurts),” Dr. Barrangou points out, “but also for limiting the presence of detrimental pathogens or spoilage microbes.”
According to Dr. Barrangou, each year food pathogens cause about 9.4 million foodborne illnesses in the United States alone. Further, he estimates that spoilage microorganisms destroy more than a quarter of the world’s food supply. He insists that CRISPR-Cas technology can benefit food science on many levels, across the food supply chain.
CRISPR-based genome editing is already being employed to increase crop hardiness and nutrition as well as improve herd genetics. Also, recent U.S. Department of Agriculture (USDA) actions suggest that the agency will exercise regulatory forbearance if advanced gene-editing technologies avoid transferring DNA from donor organisms. For example, white button mushroom and waxy corn altered by CRISPR technology have escaped USDA regulation.
Still, there is a need for oversight. “Major issues in the industry relate more to public perceptions as well as ethical concerns (particularly for human and animal work),” Dr. Barrangou reflects. “As scientists, we must remain vigilant to understand where the technology is leading and how quickly we should implement it, while continuing to engage in open and productive dialogues. I believe this amazing and powerful technology could not only feed the world, but also enable greater food safety and provide sustainability across the globe.”

The laboratory of Rodolphe Barrangou, Ph.D., at North Carolina State University focuses on CRISPR-Cas systems in bacteria, especially as they relate to industrially relevant organisms for agricultural applications. Dr. Barrangou (right) is editor-in-chief of The CRISPR Journal.
CRISPR Enhances Model Development
CRISPR technology has revolutionized the process for creating genetically modified mice, allowing for shorter timelines and, in many cases, more efficient production.
In a presentation entitled, “Utilizing CRISPR Technology to Develop Mouse Models of Human Disease,” at the Hanson Wade CRISPR 2017 Conference, David S. Grass, Ph.D., senior director of genetic engineering, genotyping, and reproductive sciences at The Jackson Laboratory (JAX), discussed the impact of CRISPR technology on the JAX Model Generation platform.
The JAX Experience
Dr. Grass highlighted the JAX team’s ability to create genetic modifications on a wide array of genetic backgrounds (important for creating changes on strains that have preclinical relevance), and he contrasted the CRISPR technology with more traditional techniques, such as homologous recombination in mouse embryonic stem (mES) cells, for creating genetic modifications.
“CRISPR technology increases the efficiency of homologous recombination such that the genetic modification can be performed in single-cell embryos, as opposed to mES cells,” said Dr. Grass. “In addition to decreasing the timelines and the cost for creating the genetically modified mice, this allows for the work to be performed directly on different genetic backgrounds for which robust mES cells don’t exist, avoiding extensive backcrossing from the strain the mutation was made on to the preferred background strain.”
Case Studies
Case studies were presented for two projects performed for Cat Lutz, Ph.D., an investigator at JAX. For one, an allelic series was created for familial amyotrophic lateral sclerosis (FALS) using an oligo-mediated knockin strategy to create different SNPs in the endogenous TUBA4 gene that are similar to those associated with patients with FALS.
The other project involved the creation of a model for Friedreich’s ataxia, for which a conditional Frataxin null allele was created using a double-stranded DNA plasmid-mediated knockin strategy.
The processes for validating the mice also were discussed. These include PCR and sequence analysis, designed to ensure that the desired mutation exists in the genome and has occurred at the targeted locus.