Cutting-edge technologies celebrate their disruptiveness, but only for a while. They actually yearn to be domesticated. For example, when single-cell RNA sequencing (scRNA-seq) appeared, it exposed the limitations of bulk RNA sequencing. The upstart technology even won a measure of respectability. In fact, it became indispensable, at least to scientists interested in cataloging gene expression data for individual cells. Still, scRNA-seq can’t capture how gene expression data might reflect the spatial relationships among multiple cells, and it can’t deliver tissue-level insights.
Spatial transcriptomics—or “spatial,” as it is commonly known—may help scRNA-seq finish the revolution it started. Spatial is nothing less than the genome-wide readout of gene expression at the tissue scale, suggests Fei Chen, PhD, core institute member at the Broad Institute and co-developer of Slide-seq, a spatial technique. Spatial, Chen declares, gives us “a new way to look at tissue biology.”
For a long time, scientists described tissues using traditional pathology. With this approach, Chen notes, “We have never had the molecular understanding.” But now, with spatial, molecular measurements are no longer restricted to dissociated single cells. The technology can dig into key questions underlying how tissues function by analyzing the mRNA expression profiles, or transcriptomes, of single cells without removing them from the context of their tissue.
Spatial, Chen adds, will allow researchers to answer questions that used to be unanswerable: What cells are interacting with each other and at what range? How are these cells organized, and how do their organizational patterns get disrupted during disease? How does gene expression change as a function of space?
Researchers working on these questions are uncovering new aspects of cancer, neuroscience, stem cell differentiation, and more. GEN spoke with several researchers, including pioneers of spatial technology rapidly being commercialized, to understand the questions they are asking and the role they see spatial playing in the future of biological research.
A pathologist, a spatial expert, and a wise mentor
Sherman Weissman, MD, a veteran professor of genetics at Yale School of Medicine, has enjoyed a distinguished career since graduating from Harvard Medical School in 1955, mentoring numerous standout researchers including Francis Collins, MD, PhD, the director of the National Institutes of Health. Weissman’s wisdom was recently put into action when he introduced two of his junior colleagues to each other and suggested a collaboration.

One colleague is Samuel Katz, MD, PhD, a pathologist who has studied blood disorders at Yale for almost a decade; the other colleague is Siyuan (Steven) Wang, PhD, a Yale faculty newcomer who is an expert in spatial biology. They agreed to Weissman’s suggestion, marrying expertise in histology and disease with expertise in a cutting-edge technique. Working together, the scientists soon achieved a first in spatial biology. Specifically, they applied spatial technology to study hematopoiesis in the fetal liver.
Katz and Wang used a spatial technology called MERFISH (multiplexed error-robust fluorescence in situ hybridization). Wang was already well acquainted with the technology, having been an author of the original MERFISH paper (Chen et al. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 2015; 348(6233)). Wang co-developed the technology when he was a postdoctoral fellow at the Massachusetts Institute of Technology (MIT), in the laboratory of Xiaowei Zhuang, PhD.

The technology has been commercialized by Vizgen, but Wang uses a home-built system. Some other labs have built their own MERFISH systems, but doing so isn’t easy, Wang explains. It requires computation and engineering expertise in everything from instrumentation to software. Although Wang’s name is on the MERFISH patent, he is not involved with Vizgen—by choice. He prefers his academic freedom and working with his students.
Katz’s passions are pathology, which he describes as “a visual appreciation of diseases,” and the liver—especially the special niche of the fetal liver where hematopoiesis occurs at rapid pace. Because conventional single-cell transcriptomics techniques require the dissociation of cells, it is challenging to analyze niche information. Katz turned to spatial to try to understand the organization of the fetal liver niche and uncover factors that control hematopoiesis.

The fetal liver is a particularly challenging space to study spatial organization. It is very homogenous, in part because it hasn’t fully developed yet. The cells are “all packed on top of each other,” notes Katz. But Wang thinks that there may be more organization than is appreciated and proposes the presence of hidden, undiscovered, biologically meaningful spatial information.
Hematopoietic stem cells (HSCs) are in a “vast sea of other cell types” in the liver. Less than 1% of the cells in the liver are HSCs; they are in a morass of hepatocytes, erythroid progenitors, endothelial cells, macrophages, and megakaryocytes. There have been hints that HSCs are influenced by the signaling molecules released by nearby cells. But spatial information is critical to understanding which cells (and signaling molecules) might be regulating the behavior of HSCs.
The Yale team’s spatial work indicated that endothelial cells actually touch HSCs. Indeed, Wang and Katz are exploring whether every HSC touches an endothelial cell. They also plan to analyze how some of the characteristics of HSCs—differentiation and proliferation capability—are determined by the gene expression profile of their nearest neighbor.
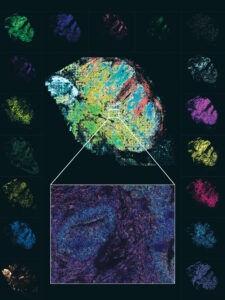
One advantage of MERFISH over other spatial technologies is its impressive spatial resolution. Going beyond the single cell, MERFISH offers single-molecule resolution that may be important in a system such as the brain, where subcellular localization can be biologically relevant. Another advantage of MERFISH, notes Katz, is the ability to detect genes that are expressed at low levels. In the fetal liver study, this was “absolutely critical” because the transcription factors have low expression levels, and they might have been missed using a sequencing-based method.
Katz and Wang now have a trove of data to work with, allowing them to explore more biology in this system—the spatial arrangements of fetal liver cell types, the preferential neighboring relationships, and the underlying ligand and receptor relationships. Also, Weissman has another feather in his cap.
Communities of cancer cells
Most people consider cancer as a “disorganized, jumbled up bag of cells,” explains Alex Swarbrick, PhD, laboratory head in the tumor progression laboratory at the Garvan Institute of Medical Research in Sydney, Australia. In addition, host cells have been generally considered “pretty passive” bystanders, but the needle is swinging the other way. Driven in part by the field of immuno-oncology, which has illustrated that host cells are important determinants of cancer cell behavior, researchers are focusing on tissues where spatial will play a big role.
Spatial is about much more than revealing where something is located, asserts Swarbrick. With spatial, researchers can investigate how cells relate to each other; how they are positioned within a tumor; and how those communities of cells define the etiology of disease, respond to therapy, and reflect their metastatic propensity. Spatial can deeply phenotype cells and determine states. For example, spatial may find that T cells are found next to fibroblasts. But it can also indicate that T cells are in an exhausted state and may be expressing certain receptors when adjacent to fibroblasts. There is a dimensionality to this method that allows for the development of tighter, more refined hypotheses.
In the Swarbrick laboratory, spatial abounds. By next year, his group will be doing more spatial work than single-cell work, using both 10x Genomics’ Visium and Nanostring’s GeoMX. Much of their research is done on human formalin-fixed paraffin-embedded (FFPE) tissues, and both of those platforms deliver robust performance in those tissues. But members of Swarbrick’s laboratory are “dipping their toes” into some other platforms to achieve higher cell resolution.
The Swarbrick laboratory, which focuses on breast cancer, is trying to understand the ecosystems in metastatic cancers, and how they affect the behavior of metastases. Because metastatic lesions in different areas of the body respond differently to treatments, they are asking how the ecosystems compare between, say, a metastatic cancer cell growing in the liver versus the lung.
The idea of cellular ecosystems, where interactions determine the phenotype, has been largely missing in cancer genomics, although there have been hints that the cells at the leading edge of a tumor look different than those in the center. But new spatial data show that cancer phenotypes are, indeed, spatially segregated. According to Swarbrick, spatial brings cancer genomics and the tumor microenvironment worlds together. It is the technology, he says, that the field has been awaiting for many years.
Slide-seq technology
Fei Chen may not be the sage that Weissman is—to be fair, he is only a third of Weissman’s age—but he is a veteran when it comes to spatial, having been involved since the beginning about eight years ago. During his doctoral work, Chen co-developed a technique called expansion microscopy, then added the use of fluorescent in situ sequencing, in collaboration with Harvard Medical School geneticist George Church, PhD, and MIT optogenetics pioneer Ed Boyden, PhD.
Over time, Chen grew to appreciate the limitations of microscopy while becoming interested in multiplexing technologies. Imaging-based technologies, he learned, are often difficult for researchers to use. He arrived at the Broad excited to develop sequencing-based technologies—because, he notes, everyone has a sequencer. He teamed up with Evan Macosko, MD, PhD, to design a single-cell method to capture spatial information from tissues.
When Chen and Macosko went to work, the original paper on spatial transcriptomics had already been published by researchers at the Karolinska Institute and Science for Life Laboratory in Sweden (Ståhl et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 2016; 353(6294): 78–82). The technology described in this paper became the basis for 10x Genomics’ Visium platform. But the microarray spots that were used initially were quite large—roughly 100 microns. Chen wanted to develop a technique that could look at individual cells. Because, he says, it makes sense to think about biology at the single-cell level.
How Slide-seq works is “not that complicated,” according to Chen. He likens it to Visium but adds that it has “much higher resolution.” Once the array is made, performing an experiment is easy.
For the past two years, the Chen laboratory has been making arrays for its collaborators. Access to the arrays is the rate-limiting step, something Chen wants to mitigate. This could become the work of core facilities, he notes.
The other possibility “on the table” is commercialization of the technology, although that will take time. Until this possibility is realized, Chen’s laboratory will keep churning out arrays for grateful collaborators to uncover new biology. For example, the stem cell research group at Harvard led by Paulo Arlotta, PhD, has developed a single-cell atlas of the developing mouse brain. And Macosko is interested in studying neurodegenerative disorders.
Chen’s lab is also developing computational tools to address questions about relationships between different cell types. The challenge, he says, is that new computational algorithms are necessary to analyze the data.
Layers upon layers
The brain is a critical organ with an organized architecture. But Aparna Bhaduri, PhD, assistant professor of biological chemistry at the David Geffen School of Medicine, UCLA, is interested in using spatial transcriptomics to add more information about the structure-function relationship in the developing human brain.

Bhaduri did her postdoctoral work in the laboratory of Arnold Kriegstein, MD, PhD, professor of neurology at the University of California, San Francisco, studying the developing human cortex. Using both scRNA-seq and spatial, Bhaduri developed an atlas of the developing human cortex.
Tissue size in the developing human brain is much larger than that in the mouse. Compared to imaging using a confocal microscope, using spatial is much quicker. Bhaduri was able to analyze eight brain sections using the Esper, the synthetic aperture optics-based spatial platform sold by Rebus Biosystems. The imaging took about 48 hours per section—a quick turnaround time to generate spatial data. Speed is one of the features of the Rebus Esper, which may be especially applicable for researchers who want to study neurodegenerative diseases. Such work requires the imaging of different parts of the brain.
A big question for Bhaduri is whether spatial will be a major part of her research program going forward. She points to the funding difference between her small new group versus her former well-funded lab. Spatial experiments are expensive; one area where the cost is high is in making the probes. The current price means that Bhaduri can’t put spatial into her laboratory’s regular rotation of methods.
Instead, she opts for single-cell sequencing, which she calls her “bread and butter.” These experiments give her information that she knows how to interpret. One of the challenges of spatial experiments is that the large amounts of data can be hard to interpret biologically.
Bhaduri notes that 10x’s expansion of the Visium into FFPE tissues is exciting. UCLA houses massive banks of cancer tissues, which would certainly be useful. She notes that the closer Visium gets to single-cell resolution, the more exciting it is to her.
Her crystal ball says that spatial will be able to target more genes at lower cost, and that it will become the preferred way to conduct single-cell experiments—with everything spatially resolved.
She is not alone in that prediction. Spatial researchers agree that this technology is going to be much more widely used in the future. Wang forecasts that spatial platforms will be as common as confocal microscopes.
Spatial researchers are training their sights beyond transcriptomes. Using spatial, they look to combine genomic, transcriptomic, and proteomic analyses using multi-omic spatial techniques. As a clinician, Katz can see a place for spatial omics on the diagnostic frontier. The biggest commonality between spatial researchers? They see a future full of possibilities and have an awareness that they are using a method that is going to bring biological research to the threshold of a new age.

