May 1, 2015 (Vol. 35, No. 9)
In The Land of Bewilderingly Diverse Expression Products, Even Semi-Uniform Batchwork Is King
Uniformity is king when it comes to glycoforms. Once the most effective, least immunogenic form is identified, bioprocessors seek processes that replicate this molecule within reasonable boundaries—the exclusion of fucosylation, maximum galactosylation, or specific sialylation patterns.
Although reliably achieving the most desirable glycoform is an abiding challenge, it never gets old. Investigators are always on the lookout for new solutions. These investigators include Michael Butler, Ph.D., a professor of animal cell technology at the University of Manitoba.
“Consistency is the first concern. Process developers want the glycosylation pattern to be the same from batch to batch,” Dr. Butler says. “When that doesn’t occur, process developers must relate those batches to what occurred during clinical trials. And next, within that level of consistency, you want the most clinically effective form of the antibody depending on the molecular basis of its clinical use.”
For example, anti-inflammatory isoforms may be required for one type of therapy, but they may be unnecessary or even undesirable for others. Higher animals natively generate “designer” glycoforms through polyclonal antibody creation, where glycoprofiles change according to in vivo needs.
Dr. Butler’s discovery relates to levels of nutrients such as glutamine and glucose. In fed-batch cultures, low levels of these substrates are preferred because this assures the most efficient energy metabolism and the lowest byproduct or impurity generation.
But very low levels, particular for glucose, impinge negatively on the type of glycosylation obtained. This is what Dr. Butler calls “macro heterogeneity,” where entire regions on the glycosylation pattern are empty. “You have to be sure in fed-batch processes to have efficient energy metabolism via low substrate levels, but also have sufficient amounts of those substrates to ensure quality,” Dr. Butler explains.
If nutrient levels and critical parameters such as dissolved oxygen are properly optimized, it is possible to assure consistent glycosylation.
The $64,000 question: Is this approach applicable to large-scale cell cultures?
Dr. Butler believes so. Results at the 5 L lab scale, which he employs, are generally conserved to at least 100 L provided controls are maintained over pH, temperature, and oxygen transfer. Dr. Butler has used batch, fed-batch, and chemostat cultures to test his idea. Although not widely used at manufacturing scale, chemostat cultures allow adjustment of one process variable at a time to isolate conditions affecting critical quality attributes.
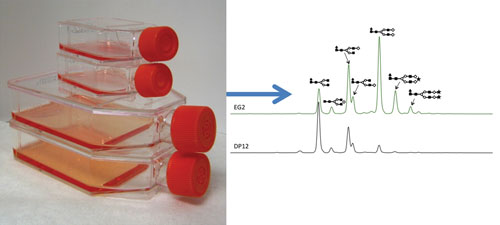
Scientists at the University of Manitoba examined two monoclonal antibodies and found differences in their glycan profiles. EG2, a chimeric llama-human antibody, was shown to have specificity to EGFR; DP12, a humanized antibody, specificity to IL-8. Both were produced from CHO cells grown in the same serum-free media (Biogro). (For details, see J. Biotechnol. 2014. Jan. 20; 170: 17–27.)
Overcoming Limitations
Achieving high titers has become mandatory for antibody manufacturing, yet companies relying on fed-batch processes still encounter production limitations. Conventional fed-batch culture utilizes large feed additions resulting in diluted final product, or incorporation of concentrated feeds that requires the addition of acidic and basic subgroups, leading to difficult-to-manage production at large scale.
Thermo Fisher Scientific has introduced a single-part dry-format feed media for CHO bioprocesses that hydrates in reduced liquid volumes without the need for pH adjustment. The product, EfficientFeed™, is enabled by proprietary technology that allows delivery of higher concentrations of difficult-to-solubilize components.
“Our approach addresses several challenges associated with large-scale fed-batch manufacturing,” says Rebecca Moore, Ph.D., senior scientist for bioproduction. These challenges include bioreactor working volume restrictions, safety concerns with large volumes of high-pH solutions, lengthy fluid transfer times, and limited storage space.
“It also diminishes complications accompanying pH-adjusted solutions,” adds Dr. Moore. The complications include complicated preparation requirements, additional pH control procedures, excess osmolality from salt formed through pH adjustments, supply chain pressures from use of short-shelf-life materials, and the inability to combine feed solutions without component precipitation.
Dr. Moore cites this feed product as an example of process intensification by virtue of a two- to three-fold reduction in feed volume, and the potential for a substantial increase in production capacity resulting from the delivery of proportionately more nutritional content with increased volumes of super-concentrated feeds. “Eliminating pH adjustment steps also meets the definition of intensification,” Dr. Moore explains.
These materials can achieve stable concentrations of 150–200 g/L at neutral pH after straightforward reconstitution in water, resulting in solutions without excess osmolality associated with pH adjustment. The simplicity of reconstitution reduces the risk of errors.
Treading Carefully with Single-Use
Single-use technology has enhanced protein-generating productivity by eliminating non-value-added activities such as cleaning and cleaning validation. While disposables are widely used for many mammalian cell lines, adoption in bacterial systems is far less common due to engineering issues related to agitation and gas exchange. For both microbial and insect culture systems, the lack of a large body of knowledge gleaned from years of experience with mammalian cell culture is telling.
Biomanufacturing with insect cells faces the same challenges, and raises the same questions that were common among mammalian culture specialists a decade ago. That insect cell cultures are already quite innovative tends to compound issues related to lack of experience.
That is beginning to change. Protein Sciences has been quite successful with its FluBlok influenza vaccine. Unlike traditional vaccines produced through the familiar, decades-old egg-based process, FluBlok employs expresSF+® insect cells to express (through baculovirus infection) an immunogenic virus surface protein, hemagglutinin, from anticipated virus strains.
The company maintains a 500 L culture facility at its headquarters in Meriden, CT, and a 2,500 L facility in Pearl River, NY. This process can either be used for seasonal flu vaccine production or pandemic flu vaccine. Both sites have traditionally conducted cell culture in stainless steel bioreactors.
Jamal Meghrous, Ph.D., senior scientist, tells GEN that for hemagglutinin expression, single-use bioreactors work as well as fixed-tank reactors, at least at bench scale. At the same time, he cites his company’s significant investment in stainless steel.
“Significant development work needs to be done before switching to single use,” Dr. Meghrous advises. “Other issues exist that may affect cell growth and productivity.”
Nevertheless, the company recently integrated single-use processing for virus production. While upstream of hemagglutinin manufacture, these moves represent a positive step toward single-use manufacturing for insect cell systems.
Up and Coming: ADCs
Old protein production challenges never die, they are simply replaced by new ones. The emergence of antibody-drug conjugates (ADCs) perfectly illustrates this point.
Bioconjugation has been applied successfully and commercially to all three major biological product types: monoclonal antibodies, vaccines, and recombinant proteins. ADCs are of particular interest because of the precise targeting and long circulating half-life conferred by the antibody component, and the cytotoxic capabilities of the conjugated drug. With approximately 40 ADCs currently in development, one could say that therapeutic chimera has breathed new life into monoclonal antibodies.
Developed by Catalent Biologics, the SMARTag™ platform for manufacturing ADCs incorporates the three major ADC components—monoclonal antibody, linker, and drug.
“Each of these pieces must be carefully considered when designing an ADC to enhance its overall in vitro and in vivo properties,” says Penelope M. Drake, Ph.D., biology group leader. The SMARTag site-specific conjugation chemistry yields homogeneous, stable ADCs with strong antitumor effects, long half-lives, and reduced toxicity compared with conventional ADCs.
SMARTag also affords the opportunity to explore structure-activity relationships surrounding payload and linker composition, according to Dr. Drake: “The conjugation chemistry is compatible with different classes of payloads and toxins with different modes of action, including tubulin disruptors and DNA alkylators.” And the versatile chemistry is compatible with cleavable and noncleavable linker systems, as well as a variety of stable linker components.
Dr. Drake cites work by Seattle Genetics demonstrating that the stoichiometry of drug loading significantly influences a drug’s pharmacokinetics, and with it efficacy and toxicity. It is now recognized that for some ADCs, a drug-antibody ratio (DAR) of 4 is more potent than a DAR of 2, and that 4 was comparably effective but better tolerated than a DAR of 8.
“It is therefore important to carefully consider DAR when designing an ADC to achieve optimal drug loading to maximize therapeutic index,” Dr. Drake concludes.
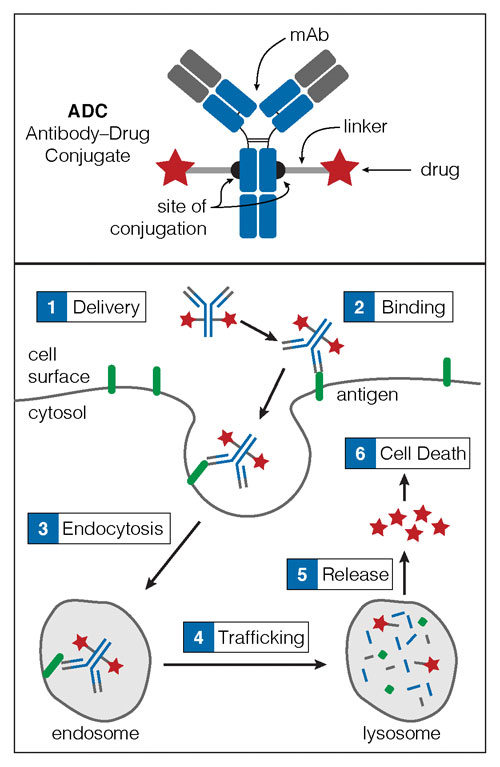
An ADC consists of a monoclonal antibody (mAb), a membrane-permeable payload, and a chemical linker (top). The variable region of the mAb (gray) is the region that binds to the cell surface antigen (bottom). Once the ADC is delivered (1), it binds (2) and is internalized via endocytosis (3). The ADC then trafficks to the lysosome (4), where lysosomal proteases degrade the ADC into its component parts. The small molecule drug payload is released into the cytoplasm (5) and accesses molecular targets, inducing cell death (6). [Catalent Biologics]
Control via New Chemistry
Seattle Genetics’ controlled conjugation strategies encompass several aspects of ADC manufacture and product control, including process consistency and robustness, and control of product quality. The goal of controlled or site-specific conjugation is greater homogeneity of the ADC product.
Essential to Seattle Genetics’ ADC conjugate technology are the stable linkers and synthetic cytotoxic agents. In preclinical models, the company’s linkers are up to 10 times more stable in blood than conventional linkers.
The lead cytotoxic agents are the auristatins, which constitute a class of microtubule-disruptors. These agents include monomethyl auristatin E and monomethyl auristatin F. The auristatins are 100 to 1,000 times as potent as traditional chemotherapy drugs. Seattle Genetics is also investigating another ADC technology that uses an extremely potent cytotoxic agent, a pyrrolobenzodiazepine dimer.
Because both linker and cytotoxic agents are synthetic, this ADC technology is readily scalable. According to the company, this represents an improvement over natural product drugs which are more expensive to make.
“The ADC supply chain is complex,” comments Michael Sun, Ph.D., director of clinical manufacturing. “The manufacture of the antibody and small molecule components require different production facilities and skill sets.”
Conjugation of components to produce an ADC presents another unique set of requirements related to the combination of biologics and small molecules. Once the need for special safety protection (due to the high potency of the small molecule and the ADC) is factored in, the complexity of production facilities, each with its specialized capabilities, becomes apparent.
“Coordinating the activities across the different parts of the supply chain can be difficult,” explains Dr. Sun. “And the total time required for production is quite a bit longer than for a typical biologic product.”
Achieving coordination becomes even more difficult when a contract manufacturing organization (CMO) is involved.
“A good synergistic strategy followed by innovative companies involves leveraging the depth of in-house process and analytical development with the manufacturing and high potency capability of CMOs,” Dr. Sun continues. Under such a strategy, process and analytical development is performed in-house, and technology is transferred to CMOs for manufacturing. “This keeps the expertise and subject matter experts in-house, where those assets are most valuable, for example, in regulatory submissions and inspections, while outsourcing manufacturing activities without the infrastructure investment.”
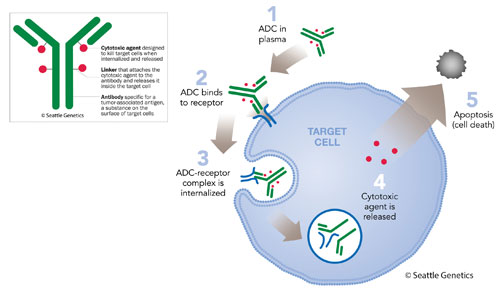
Seattle Genetics’ antibody-drug conjugate (ADC) technology employs a monoclonal antibody specific for a tumor-associated antigen, plus synthetic cytotoxic agents connected by stable linker systems (see the inset). These components are designed to securely bind the cytotoxic agent to the antibody and then release the agent within the targeted cell (see the main image).


