
Simple paper-based test devices, including home pregnancy tests, rely on lateral flow. More sophisticated devices introduce hydrophobic/hydrophilic architectures. The device shown here relies on lateral flow, but it also incorporates a stacking flow design to create multiple flow paths and, ultimately, detect dengue antibodies from saliva samples. The device’s designers at A*STAR in Singapore say that the device could be adapted to detect other infectious diseases such as HIV and syphilis.
Backed by increasingly sophisticated technology, paper-based immunoassays may become the common coin of antibody-antigen quantitation. They are already poised for wide circulation in resource-constrained environments. Eventually, they may become the preferred approach in many other settings, including those now dominated by traditional immunoassays. Although traditional immunoassays often qualify as “gold standard” tests, many of them are inconvenient, requiring complex multistep protocols, expensive equipment and reagents, and trained personnel.
If these inconveniences could be avoided with sufficiently advanced paper-based immunoassays, users in both the developing and developed worlds could accomplish various kinds of testing—pharmaceutical analysis, clinical diagnostics, environmental monitoring, and food testing—without having to rely on large, centralized laboratories. In many situations, decentralized or point-of-use testing could save time and money while delivering adequate functionality.
Fluidic circuits on paper
Many commercial devices that measure parameters or metabolites in body fluids are limited because they require end users to load individual reagents on the device in a specific order. “That type of platform requires a preprocessing step and is not autonomous,” says Mohammad Faghri, PhD, a professor of mechanical engineering at the University of Rhode Island. “But we could make it autonomous with our platform.”
Faghri is referring to a platform that is being developed by Labonachip, a startup company that he co-founded and currently serves as scientific adviser. The platform, which incorporates paper-based microfluidic valve technology, is designed to perform multistep enzyme-linked immunosorbent assays.
“The uniqueness of our platform is that it contains a fluidic circuit on paper that can sequentially add reagents to perform very complex and sophisticated protocols,” Faghri asserts. The platform’s proprietary technology creates a fluidic diode that ensures unidirectional transport on paper or paper-like materials.

Paper-based devices are often thought to be suitable for use in low-resource settings or in poor countries, but at Labonachip, these devices are also appreciated for their potential in developed countries, indicates Constantine Anagnostopoulos, PhD, the company’s co-founder and president. Anagnostopoulos, who is also an adjunct professor of mechanical engineering at the University of Rhode Island, suggests that healthcare tests that used to be confined to centralized labs are moving into doctors’ office or mini-clinics.
“We constructed a fluidic circuit that could autonomously and sequentially load several reagents,” declares Faghri. In subsequent research efforts, Faghri, Anagnostopoulos, and colleagues incorporated this paper-based microfluidic valve technology into a platform that uses three-dimensional micropatterned techniques and test strips to generate a new type of microfluidic device. In this system, the sample fluid activates the subsequent flow of reagents in a predetermined sequence and with a predetermined delay.
In a proof-of-concept study, four valves directed the flow of three different reagents and achieved a detection limit of 4.8 fm. “From the technology side, the adoption of these assays will be paced by how companies and regulatory systems will allow them to develop,” notes Faghri. “But I predict that within 20 years, instead of big labs, we will see small labs in the palm of our hands.”
Additional efforts in Faghri’s group are focusing on strategies to communicate the data by wireless technologies, providing opportunities for transferring information about disease and wellness markers from paper-based devices, through an iPhone, to healthcare providers. “I can see a parallel between the miniaturization of healthcare devices and photography,” says Anagnostopoulos. “Years ago, photography used large darkrooms and was complicated, but today the entire process has been reduced to a few seconds.”
The miniaturization of healthcare devices requires collaborations among professionals from multiple fields, including biologists, chemists, physicists, engineers, and computer scientists. “When we started this project more than 10 years ago, we encountered the need for professionals from all these areas,” recalls Faghri.
The technological development of any disease marker starts with developing a laboratory test to characterize its utility and validity. However, if the test is to be carried out on a microfluidic paper-based device, development must move beyond capturing biology. “Concentrations should be diluted to a level that can be detected on paper, and the chemistry should therefore be optimized,” explains Faghri. Development should also address the design of fluidic circuits, which “load various reagents in different locations and at specific times,” details Faghri, who adds that precise fluidic loading is determined by the characteristics of the material. Ultimately, through interdisciplinary collaboration, what started as a conventional laboratory reaction becomes a paper-based assay.
Paper-based miniaturized devices are attractive not only for the clinic, but also for other arenas, such as agriculture and food sciences. “They could be used to measure a molecule of interest in food or water, or in the fluids of plants and trees,” suggests Faghri.
Increasing the efficiency of target capture
One of the challenges faced by many diagnostic assays is that only modest densities of surface-bound species have been achieved. Target molecules, which are often present in very low concentrations, may be left uncaptured, potentially decreasing the binding signal and the test sensitivity. Three approaches are generally available to enhance the kinetics of target-substrate interaction:
- Increasing the abundance of the soluble antigen.
- Increasing the concentration of its immobilized binding partner.
- Improving the affinity of the binding interaction.
The second approach was used in a recent effort led by Hadley D. Sikes, PhD, associate professor of chemical engineering at MIT. Sikes and colleagues generated a bifunctional fusion protein that incorporates a cellulose-binding domain and an antigen-binding domain. The former domain enabled the high-abundance immobilization of the latter, a thermostable protein of ~9 kDa.
“Biotechnology, which has been critical in enabling our work, helped us generate a protein that is much smaller than an antibody but functions as an antibody,” reports Sikes. The smaller size of the protein allowed for a much higher density of the capture molecule on the paper. “We were able to get close to 200 picomoles immobilized on our spot,” Sikes continues. At this molar abundance, the immobilized species is present in an over 60-fold molar excess relative to the soluble antigen at the highest titer concentration.
This increase in concentration dramatically accelerated sample capture, Sikes points out. The high local concentration of the fusion protein allowed nearly 100% efficiency in capturing target molecules from dilute solution, thus improving detection sensitivity. That’s obviously a very desirable characteristic in the development of diagnostic applications. Scientists in Sikes’ group then showed that the assay functions equally well for serum, saliva, and urine samples.
In January 2018, Sikes joined the Antimicrobial Resistance Interdisciplinary Research Group (AMR IRG), a translational research and entrepreneurship initiative in the Singapore-MIT Alliance for Research and Technology (SMART). “To combat the threat of antimicrobial resistance, the National Research Foundation has invested heavily in diagnostic surveillance and in developing tests,” notes Sikes. “Having them available at very low cost is an important part of that program.”
A key focus of Sikes’ group is ensuring that the cost of the tests that are generated is maintained under 10 cents. “So far, we have been meeting that goal,” declares Sikes. The most expensive component of the test is the hydrophobic ink, which is used in paper-based assays to define the test zones where the capture protein is deposited.
“In terms of what disease applications are most relevant in the developing world, we have been working with proteins and biomarkers to diagnose tuberculosis,” says Sikes. The development of assays that can diagnose tuberculosis quickly is particularly important because existing microbial cultures for this pathogen take longer than those for most other microorganisms. In addition, the drug regimens are lengthy and cumbersome. Consequently, it is important to have some way to monitor whether treatment is effective.
Measuring hematocrit with a paper-based test
A limitation of standard clinical immunoassays is that, even though they provide information in a timely manner, most of them require many labor-intensive manipulations, such as multiple pipetting steps, temperature changes, or plate shakings—all of which require well-trained personnel and facilities. “At the other extreme is the lateral flow test, which has been around for decades and is very user-simple,” says Charles Mace, PhD, assistant professor of chemistry at Tufts University.
An example of such tests is the home pregnancy test, where a sample is deposited on the device and the results are generated automatically. “Paper-based microfluidic devices are somewhere in the middle, where one can shoot for the simplicity of the operation or the output of a lateral flow test,” notes Mace. One can also, Mace continues, attempt to program some more complex operations into the device.
These operations can take the form of more complex chemical reactions, multistep processes, or controlled timing of interactions, which bring the functionality of the platform closer to the type of test that one can perform in a clinical laboratory. “This is a great challenge because the promise and the need are really there,” Mace explains, “and the platform has the capabilities to meet these needs—it’s just that the process is not a short one.”
In recent years, Mace and colleagues have developed a thermometer-style paper-based microfluidic device for measuring the hematocrit, the proportion of the blood composed of red blood cells. “We are moving forward with tests that use blood collected from a fingerstick,” Mace declares. “In a lot of cases, this is about the blood cells themselves and less about the molecular analytes in the blood.” The device measures the distance that is traveled by red blood cells, which is inversely proportional to the hematocrit and directly related to the percentage of liquid in the blood.
The test indicates if a patient’s hematocrit is outside of the physiological range. If that value is too high or too low, there are specific interventions that may be needed. This test is very informative, but Mace suggests that it is, on its own, only one component of the more comprehensive picture of a patient’s health.
For devices and tests that provide clinically actionable information, understanding the underlying biological problems is paramount. Moreover, the patient samples that are used for these assays have a much more complex composition and are more difficult to predict than typical lab experiments with controlled conditions. “These assays have to work with real patient samples,” Mace insists, “And this is one of the things that require different disciplines to be brought together at the same time.”
Improving and refining paper-based assays
Many assays are limited by the need for multiple steps, such as the addition of buffers or the separate loading of sample and buffers on the device. “We are trying to eliminate all those steps from the process so that the burden on the user is minimized,” says Andres W. Martinez, PhD, associate professor of chemistry and biochemistry at California Polytechnic State University. “My lab focuses on the fundamental aspects of the assays in terms of how to make them work better.”
Part of the effort in Martinez’s lab is focused on making tests affordable and useful in settings outside the laboratory. “Many advances have been made,” Martinez states, “but more progress still needs to happen before it becomes reality.”
A limiting factor for many paper-based assays is the shelf life of the reagents. Ideally, these reagents should be stored in a dry form on the device so that users don’t have to carry reagent bottles. Additionally, many reagents, particularly antibodies and proteins, have limited stability and degrade over time. “One of the challenges involves finding ways to better stabilize these reagents on the device,” Martinez points out.
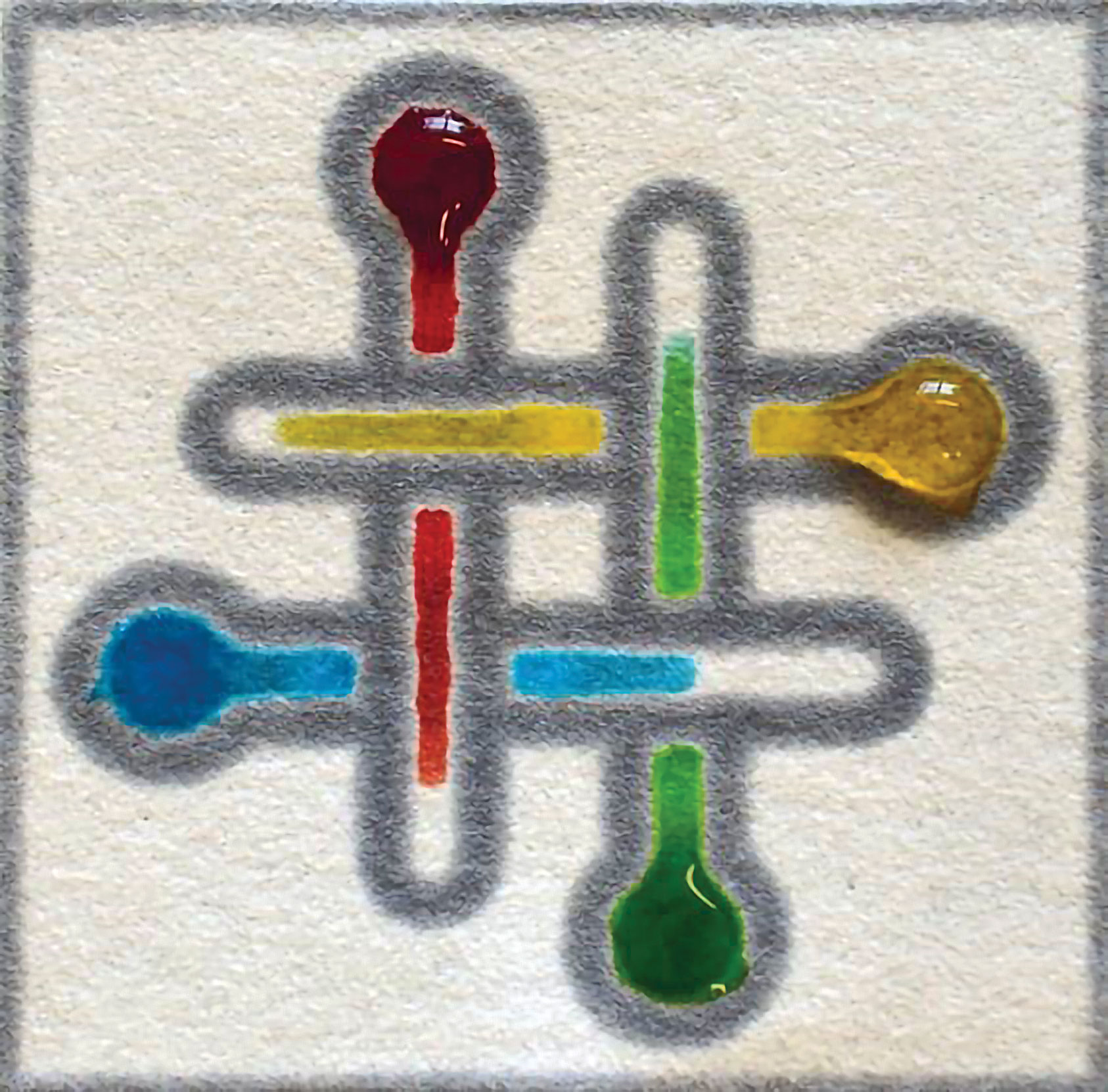
Martinez and colleagues developed a new method to generate three-dimensional paper-based microfluidic devices in which toner is used as a thermal adhesive to bind together multiple layers of paper. The driving force behind this effort was the need to generate testing devices that can be used by untrained users in remote locations or by first responders. Because this assay requires only a laser printer and a heat source, it is available for researchers in a wide array of settings and locations.
In a more recent development, Martinez and colleagues reported a method to generate high-resolution microfluidic devices by patterning paper via wax printing and then treating it with periodate and heat to miniaturize the patterned paper. This strategy allowed them to generate functional channels that are about 300-µm wide. Ultimately, the scientists demonstrated the utility of their devices for colorimetric and enzymatic assays. “Something that we struggle with is that at the laboratory scale, we don’t necessarily have the quality control that is available for industrial-scale processes,” Martinez admits. “We find a lot of batch-to-batch variability, which is a big issue when using these tests in the real world.”


