Gene editing history will have a lot to say about 2021. That year, two in vivo CRISPR-based therapies showed promise in human trials. One of the therapeutics, from Intellia Therapeutics and Regeneron Pharmaceuticals, targets the faulty gene behind transthyretin amyloidosis. The companies reported results from clinical trials showing that a month after the new CRISPR-based therapy commenced, transthyretin gene expression fell by 96%. Separately, Vertex Pharmaceuticals and CRISPR Therapeutics shared unpublished data from a small clinical trial in sickle-cell disease and β-thalassemia that showed a CRISPR-based therapy had brought about dramatic and long-lasting improvements in patients with these diseases.
Although 2021 was an outstanding year for gene editing, 2022 could end up being even more notable. If not, there’s always 2023—or another year soon. After all, gene editing keeps improving. For example, CRISPR-based systems are being enhanced in various ways. There are multiplex CRISPR systems, bioinformatically informed CRISPR systems, and CRISPR systems that incorporate customized Cas enzymes.
In addition to CRISPR systems, there are systems based on homing nucleases, or meganucleases—which, despite “mega,” are smaller than Cas enzymes and hence easier to pack into common delivery vehicles. There are also RNA-based “gene writers.” These systems incorporate retrotransposon elements to facilitate the introduction of large-scale changes to the genome.
As the Cas-oriented and non-Cas-oriented systems mature, today’s gene editing applications will come to be seen as low-hanging fruit. Gene editing systems will be optimized to treat a broader range of disease indications, and gene editing agents will reach organs and tissues of the body beyond easily accessible targets such as the eye and liver.
Proviral-DNA-flanking gRNAs
Excision BioTherapeutics uses its CRISPR-based platform to attack viral infections in the human body by snipping viral DNA out of the host genome. The most advanced product in its pipeline is EBT-101. This is a Cas9 dual-guide RNA system that is packaged within an adeno-associated viral vector, and it is being developed as an HIV treatment. Excision has successfully removed HIV genomes from animals, functionally curing the animals of the infection.
A cornerstone of Excision’s platform, which complements the CRISPR-Cas9 technology, is a bioinformatics approach to designing guide RNAs. By analyzing public and private viral databases, the company is able to choose target sequences that don’t have similar sites in the human genome.
“The viral target sites are very orthogonal to the human genome, meaning that the number of output sites in the genome are much rarer than when it targets human genes,” says Thomas J. Cradick, PhD, chief scientific officer of Excision. After these output sites are identified, the company can find ways of making two or three cuts to delete very large sections of proviral DNA. “By making these large deletions,” Cardick explains, “we substantially decrease the chance of viral escape for HIV.”
Excision’s goal is to develop “one and done” treatments for viral infections. In addition to developing treatments for HIV infection, the company is also developing treatments for JC virus (a virus that causes multifocal leukoencephalopathy), herpes simplex virus, and hepatitis B virus infections. Cradick says that Excision is looking to develop treatments for diseases that affect larger populations, treatments that the company hopes can be administered outside of highly specialized medical centers.
“We’ve been hearing [that people are excited] about our program for HIV,” Cradick says. “If there are patient populations that may not get the latest in retroviral therapy, we will address them if we’re able to scale production and move forward.”
Predictive design–multiplex editing technology
Agriculture poses many challenges. It is one of the largest contributors to greenhouse gases. It consumes a great deal of water. And it relies on nitrogen fertilizers, which run off agricultural fields and pollute waterways and coastal regions. If these problems are to be addressed, agriculture will need to become more sustainable.
Enter Inari, an agricultural biotech company. To help agriculture consume fewer resources and become more productive, the company is engineering seeds with environmentally friendly traits.
Inari’s approach starts with machine learning, probing the genome of the target crop to understand where changes can be made to help the crop acquire desirable traits. “In other words, we create a blueprint that shows us where we need to make changes,” says Ponsi Trivisvavet, chief executive officer of Inari. That blueprint results in many target sites, which Inari edits simultaneously using its proprietary platform.
“Because plants are so complex, there’s absolutely no way to make changes in one gene to solve the complex problems,” Trivisvavet explains. For example, a plant may need to increase the expression of several genes just to reduce its consumption of water.
Trivisvavet says that Inari invests about 50:50 in the “hardware” and “software” sides of the science, the hardware being the gene editing machinery. The hardware and software work together synergistically, with results from the laboratory feeding back into the knowledge base to make predictions for the next round of product development.
Trivisvavet does not specify exactly how many genes Inari can edit simultaneously using its CRISPR-based platform other than “it’s a large number.”
Custom CRISPR enzymes
The CRISPR-Cas9 gene editing system is derived from bacterial immune systems that evolved over millions of years for the purpose of protecting the cell from invasion by a virus or other hostile nucleic acids. In its original form, however, the system is not particularly suitable for genetic engineering purposes. It’s “useful starting material,” says Benjamin Oakes, PhD, CEO of Scribe Therapeutics. “But by no means does that make it a genome editing scalpel.” He adds that the characteristics of a CRISPR-based therapeutic are bound to be very different from those of a CRISPR immune system.
Scribe was founded in 2018 with the goal of engineering CRISPR-based gene editing tools for “exquisite” activity, specificity, targeting range, and delivery in a single package. Rather than use more conventional CRISPR-Cas9 systems, Scribe scientists use modified versions of CasX enzymes. (CasX, a placeholder name, stands for a distinct family of RNA-guided genome editors. An alternative name is Cas12e.)
Scribe scientists hone CasX enzymes so that they acquire characteristics such as enhanced specificity. Oakes describes this as an “engineering first” philosophy, where the desired properties of the system are chosen in advance and intentionally designed, rather than being left to chance or nature.
Scribe has taken steps to address diseases of significant unmet medical need. For example, the company has entered a research collaboration with Biogen to develop and commercialize CRISPR-based therapies that address an underlying genetic cause of amyotrophic lateral sclerosis. Under the terms of the collaboration, Scribe will receive $15 million upfront and is eligible for more than $400 million in potential development and commercial milestone payments. The company has also raised $100 million in a Series B financing round to further develop its “CRISPR by Design” platform and pipeline of genetic medicines.
Scribe has already begun testing its systems in mouse models of neurological and neurodegenerative disease. “We see, in some instances, really exciting extensions in lifespan,” remarks Oakes, adding that the company will likely begin to publicly share data on that in late 2022 or early 2023.
Oakes believes researchers will increasingly improve on CRISPR “classic” in 2022 and beyond: “People are starting to realize with the broader application of CRISPR just how important it is to really take these bacterial immune systems, melt them down, and reforge them into genome editing scalpels.”
Homing endonucleases
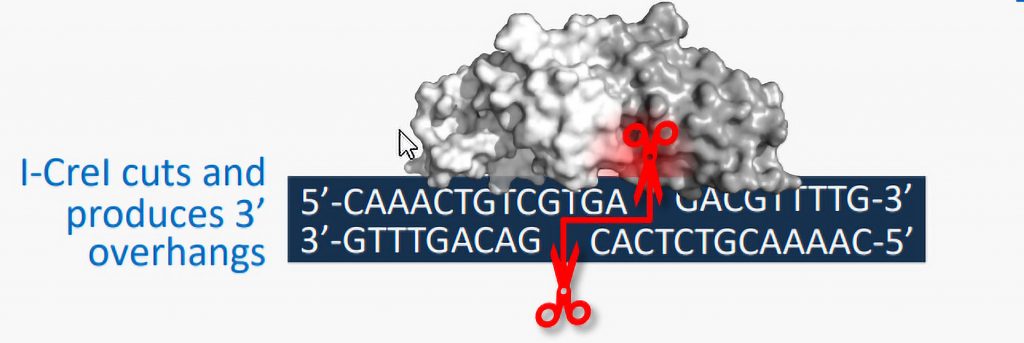
Instead of elaborating on one of the “big three” gene editing approaches (Cas9 nucleases, zinc finger nucleases, and transcription activator-like effector nucleases), Precision BioSciences has built a distinctive system that the company calls ARCUS. It is based on I-CreI, a naturally occurring genome editing enzyme from the algae Chlamydomonas rheinhardtii. According to Derek Jantz, PhD, chief scientific officer of Precision BioSciences, the ARCUS platform offers several advantages:
- It is able to turn itself off after making a gene edit, allowing more control of off-target editing.
- It creates a 3′ overhang when it cuts DNA, meaning it doesn’t cut both strands exactly in the same place. That overhang then signals the cell to perform homology-directed repair on the break, resulting in a clean and efficient insertion of DNA at the target location.
- It is the smallest of the current crop of gene editing technologies. At only about a thousand bases, ARCUS easily fits inside an AAV vector. That gives it the ability to be delivered to a variety of organs and tissues, unlike systems using lipid nanoparticle delivery vehicles, which go directly to the liver.
“[The liver] is where all of the CRISPR companies are running,” Jantz points out. “They’re pursuing gene editing in the liver with lipid nanoparticles. But we’re thinking about what’s on the horizon. The brain? Muscle? Bone marrow?”
Jantz says that multiple ARCUS systems can fit inside an AAV vector: “A number of our programs, particularly our Duchenne muscular dystrophy program, take advantage of that small size. It’s sort of like FedEx. The smaller the package is, the easier it is to deliver, and the more options you have available.”
Precision BioSciences has programs in ex vivo and in vivo gene editing. Its first in vivo gene editing program targets the PCSK9 gene for familial hypercholesterolemia and is expected to produce a therapy that will compete directly with Verve Therapeutics’ CRISPR-based gene knockout therapy. Precision BioSciences is also preparing to file an investigational new drug application in 2023 for the company’s primary hyperoxaluria type I program, and another in 2024 for a chronic hepatitis B program. On the ex vivo side, the company has allogeneic chimeric antigen receptor T-cell immunotherapy programs in its pipeline for cancers including non-Hodgkin lymphoma, B-cell acute lymphoblastic leukemia, and others.
RNA-based gene writers
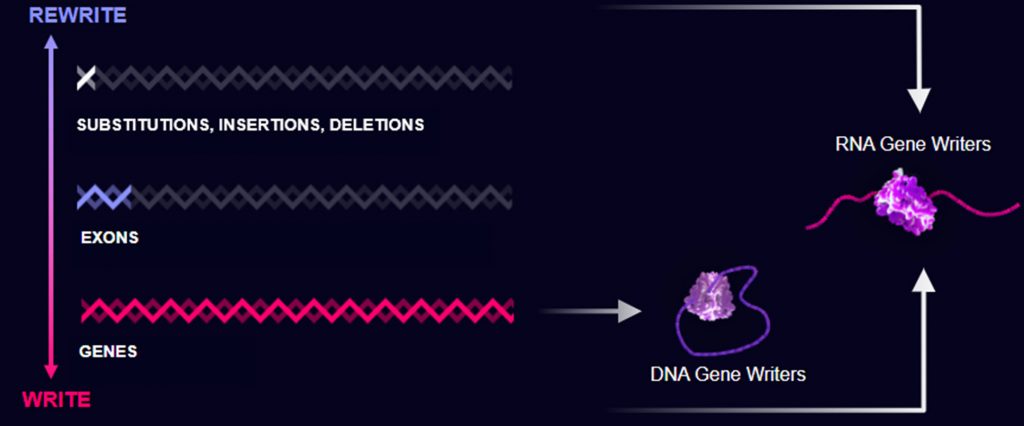
When conventional gene editing tools are used, making significant alterations to the genome is difficult. If large changes are to be made more easily, different tools will be needed. Such tools are being developed by Tessera Therapeutics, a company that is using mobile genetic elements called retrotransposons. Retrotransposons copy a sequence of RNA into new DNA. They literally write new genes.
Tessera uses a retrotransposon technology called target-primed reverse transcription to write whole genes into the genome. The technology can also make changes to a single base pair or multiple base pairs and insert or delete DNA. And Tessera can do all of this using RNA, which has been widely accepted as a therapeutic tool now that the COVID-19 mRNA vaccines have been so successful.
“It’s pretty crazy to think that RNA therapies have probably been administered to more people now than have antibody therapies,” marvels Jake Rubens, PhD, chief scientific officer at Tessera. “Using RNA, we think we may be able to scale our medicines to reach patients in parts of the world where the healthcare systems could never tolerate the cost of making viral vectors, and potentially expand from rare disease populations to more common diseases.”
Rubens says that Tessera is focused on diseases with high unmet need, on rare diseases, and on oncology. Tessera has formed a relationship with the Cystic Fibrosis Foundation to apply the company’s technology to the disease by inserting a whole copy of the cystic fibrosis gene into the genome, and potentially to also rewrite a common mutation of the gene.
The lung is an example of an organ that poses difficulties for the delivery of genetic medicine technologies, Rubens says. Nonetheless, he is optimistic about developing a cystic fibrosis treatment. He declares, “We feel the technology has matured to the point where we should take a shot on goal.”

