Recent collaborations and financing deals involving small research companies and big pharmaceutical firms are focused on next-generation cancer therapies. This activity is exemplified by widespread efforts within the industry to develop antibody and T cell–based therapies in the fight to control and perhaps defeat cancer.
Large pharmaceutical companies such as Eli Lilly and Company, Bristol Myers Squibb (BMS), and Takeda Pharmaceutical formed collaborations with smaller research teams during the first months of 2021. These collaborations portend significant advances in therapeutics that can deliver durable and long-term patient responses, as well as in therapeutics that can take on more complex cancers such as lung cancer and other solid malignancies.

Likewise, private investment firms have invested aggressively in biotech cancer companies. This article looks at the promise these investors see in next-generation oncology therapeutics and the types of therapies we might see as we approach 2030.
Investor interest
One of the big winners in the oncology space is Scorpion Therapeutics, a precision oncology company. Claiming to have developed capabilities across multiple fields of translational medicine, chemical biology, medicinal chemistry, and data science, Scorpion has attracted considerable investor interest. Last January, the company indicated that it had closed an oversubscribed round of Series B financing that raised $162 million. Scorpion has raised a total of $270 million since its founding in the first quarter of 2020.
“We have assembled a series of capabilities that allow us to prosecute whatever target we wish to work on,” says Gary D. Glick, PhD, Scorpion’s president and CEO. The financing will be used to support Scorpion’s “drug hunting engine,” which the company describes as encompassing target discovery, next-generation chemistry, and precision medicine technologies. The financing will also help the company develop a pipeline that addresses high-value oncogenes, “undruggable” cancer targets, and novel targets. Finally, the financing will enable Scorpion to move from temporary spaces in Boston to a more permanent location later in 2021.
Glick, who recalls that biotech funding was relatively meager just 10 years ago, is a little surprised at the financing Scorpion has secured. But he also believes that the financing “reflects where the company has moved in terms of its internal pipeline and portfolio.” According to Glick, Scorpion is focusing on using small molecules in programs for high-value targets where there are no known solutions, as well as on discovering and drugging targets that—according to Scorpion’s own data mining exercises—have the potential to expand precision oncology into very large patient populations where good solutions have yet to be found.
Glick sees significant opportunities in oncogenic drivers that have yet to be fully validated. For example, he maintains that the ability to identify novel targets, particularly high-profile intracellular targets, and to drug those with small molecules, holds “tremendous potential.”
Beyond targeting
Large companies often partner with small companies to keep pace with fast-paced technological developments. Such is the case with immuno-oncology. In January, BMS and ArsenalBio announced that they had formed a multiprogram discovery collaboration to advance next-generation T-cell therapies for the treatment of solid tumors.
This collaboration was just the latest of BMS’s moves in immuno-oncology. Earlier, the company had acquired Celgene, the parent company of Juno Therapeutics, to bolster its capabilities in cell therapy. BMS had also formed a collaboration with Bluebird Bio to produce a biologic for treatment of multiple myeloma.
BMS’s interest in ArsenalBio is centered around the smaller company’s development of a toolbox for modifying and manipulating cells with CRISPR and other gene editing technologies. The companies indicated that AresenalBio is expected to deploy a “full stack of synthetic biology compositions to build programmable cell therapy product candidates, composed of its PrimeR™ logic gates, CARchitecture™ derived gene expression controls, and CellFoundry™ mediated nonviral manufacturing.” The plan is to achieve controlled modification of the T-cell genome.
The collaboration with BMS is only one of many collaborations envisioned by ArsenalBio’s CEO Ken Drazan, PhD. He expects ArsenalBio will have many opportunities to leverage its arsenal of manufactured biological codes.
“Solid tumors are hostile to the invasion of T cells,” Drazan points out. “We might need to weaponize T-cell medicines in more advanced ways than just [providing] a targeting system.”
First-generation immuno-oncology approaches focused on making a single modification or a limited number of modifications to the genome of the T cell using viral vectors to deliver nucleic acids. However, viral vectors have limited payload capacity. And so, says Drazan, “drug developers are limited [with respect] to the number of instructions one can write.”
ArsenalBio’s core expertise is to use CRISPR technology to open up the entire genome of the T cell and allow for strategic placement of gene edits. The focus is on edits that create more offense, more defense, and more deliverables at the site of the tumor.
“We have a mission to develop medicines for patients,” Drazan declares. “What we are trying to create at ArsenalBio is something that is very iterative—a learning model that comes from the kinds of data that we and others generate.”
For now, ArsenalBio is focused on producing autologous T-cell therapies that are effective against traditionally therapy-resistant solid tumors, and that are safe, efficacious, and durable. In the future, ArsenalBio could work with a larger universe of partners to create generalized solutions for specific tumor indications through control of the T-cell genome.
Liberating the immune system
In another growth move, Takeda has expanded into solid tumors with a concerted effort over the past four and a half years, under the leadership of drug hunter Loïc Vincent, PhD, head of the company’s oncology drug discovery and immunology units, to shift its cancer therapy work toward transformative immuno-oncology approaches. Takeda’s previous investments in cancer therapy included the 2008 acquisition of Millennium Pharmaceuticals (and its multiple myeloma drug Velcade) and the 2017 acquisition of Ariad Pharmaceuticals (and its lung cancer therapies).
Takeda’s immuno-oncology R&D is focused on two pillars: a “cold to hot” pillar, which supports the goal of turning a non-immunogenic tumor microenvironment into an immunogenic state, and a “redirected immunity” pillar, which supports the direct killing of tumors by immune cells.
“We cannot beat cancer alone,” says Vincent, echoing a Takeda internal mission statement focused on developing strong industry partnerships to explore underexplored biological models and technologies. Takeda has developed 20-plus oncology partnerships in the past five years, including major collaborations with MD Anderson Cancer Center (to develop therapies incorporating chimeric antigen receptor–equipped natural killer cells) and a collaboration with Gamma Delta Therapeutics (to develop therapies incorporating γδ T cells). Both collaborations seek to harness innate immunity with curative intent via breakthrough technologies.

Other strong pipeline products include TAK-981, a first-in-class SUMOylation inhibitor for use against hematological and solid tumor cancers (now in Phase I); TAK-676, a STING agonist for use against solid tumor cancers (now in Phase I); and TAK-500, a next-generation antibody-STING drug conjugate for use against solid tumor cancers (now in late pre-clinical stage). In Takeda’s current pipeline of Phase I and preclinical assets, 70% of the candidates are being developed in partnerships with biotech firms or academic institutions.
Vincent suggests that this dramatic shift in corporate financial and research investment has resulted from the need to overcome the limitations of current immunotherapies, such as those targeting PD-1, CD-19, and other surface proteins. Targeted therapies of this kind may be defeated relatively easily by mechanisms of resistance, he suggests. In contrast, enhancing both innate and adaptive antitumoral immunity can enable a broader response, one that Vincent says can “bring together different cell types that will attack tumors in an orchestrated fashion.”
A recent collaboration with KSQ Therapeutics, for instance, is meant to extend Takeda’s search for new targets in immuno-oncology. The collaborators hope to leverage KSQ’s proprietary CRISPRomics discovery platform to systematically screen the whole genome to identify optimal novel targets.
“They looked at all types of targets and selected targets that have undergone extensive validation,” Vincent states. Although the drug candidates that are being explored by Takeda and KSQ are still in the preclinical phase, Vincent maintains that the companies “are advancing drug discovery approaches to those targets that outperformed PD-1 blockade and that can also be positioned in PD-1-resistant patients.”
The collaboration with KSQ also encompasses studies in which the CRISPRomics platform is used to identify novel targets in cells of the innate immune system, in particular natural killer cells. These studies complement Takeda’s other efforts to develop innate cell and off-the-shelf cell therapies.
Vincent sees cell therapy leveraging new engineering tools and moving from an ex vivo approach to an in situ approach in the next 5 to 10 years. He also sees more extensive use of induced pluripotent stem cells with custom modifications. “Each patient is different, and each tumor is different,” he says. “The beauty of what we and others are doing is liberating the immune system to kill the tumor.”
T-cell engagement
Another promising next-generation approach is the use of bispecific and multispecific antibodies to engage immune system elements, predominantly the T cell, at the site of cancer. To help realize this approach, Eli Lilly and Company recently agreed to pay up to $1.6 billion to Merus, a small biotech firm, to develop up to three CD3-engaging T-cell-redirecting bispecific antibody therapies. Under the terms of the agreement, Merus will lead discovery and early-stage research activities while Eli Lilly’s Loxo Oncology unit will be responsible for additional research, development, and commercialization activities.
The CEO of Merus, Bill Lundberg, MD, predicts that “novel science, novel biology, and novel ways of targeting cancer” will continue to see investment, particularly where medicines have been shown to be effective against cancer. “Although T-cell engagers are complicated, there is an approved T-cell engager,” he says. (This drug, Amgen’s Blincyto, targets both CD19 and CD3.) “[It shows] us that we can effectively treat cancers with T-cell-engaging antibodies.”
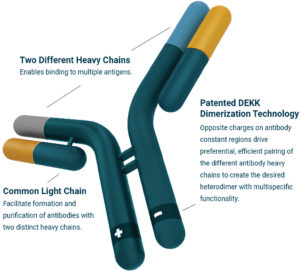
Merus develops bispecific and multispecific antibodies based on the common light chain format. Specifically, the company exploits this format in its proprietary Biclonics and Triclonics platforms to achieve true high-throughput screening of T-cell engagers called Multiclonic therapeutics.
“It is clear that bispecifics can bring the T cell to the cancer and kill [cancer cells] very effectively,” Lundberg asserts. “The question is now how to optimize the efficacy while ensuring safety.”
Merus has developed a robust panel of more than 175 unique and novel antibodies that bind to T cells via the CD3 antigen. “When you have such a large panel of T-cell-engager-type molecules,” Lundberg points out, “you can start to separate out which ones bind on the CD3 molecule and lead to T-cell activation and cancer cell killing, and also potentially improve safety with lower cytokine release.”
One of Merus’ lead clinical candidates, Zenocutuzumab (or Zeno), has been developed for patients with genetically defined cancers that have an NRG1 fusion. “Zeno acts by potently blocking the binding of the protein expressed by the NRG1 fusion to the HER3 receptor,” Lundberg reports, “preventing formation of the HER2/HER3 complex that this signaling pathway goes through and preventing cancer growth.”
Other areas where bispecific antibodies may have significant impact include lung cancer. “We are developing a medicine called MCLA-129 that simultaneously targets both the c-MET and EGFR proteins on the surface of cancer cells,” says Lundberg, “maximizing the killing of cancer cells that express both proteins while minimizing any unintended off-target effects on normal cells that typically express only one or the other protein.”
“Our common light chain technology platform solves a fundamental problem in the field of bispecific and multispecific antibodies,” Lundberg remarks. “Bispecific antibodies, in the natural, human antibody format, comprise two different heavy chains and two different light chains. These components, when expressed together in a cell, can form antibodies nine different ways.”
Lundberg asks, “How do you ensure that the cell produces the specific one of these nine forms of antibodies that you are interested in?” Then he proposes an answer, indicating that the Merus Biclonics approach is to reduce the complexity by using the same common light chain in every antibody, so the only difference between antibodies is the heavy chain.
“We ensure the preferred use of heavy chains with a proprietary charge-pairing approach,” he explains. “We believe that our key advances in bispecific antibody engineering—which enable true high-throughput screening—together with our large, novel, and diverse panel of CD3-T-cell-engaging antibodies, are what positioned us as the partner of choice for Loxo Oncology at Lilly.”