April 1, 2014 (Vol. 34, No. 7)
Antibodies have emerged as important therapeutic agents for cancer. Many clinically relevant mechanisms of action are exploited in creative strategies aiming to engage the host immune system in cancer treatment.
This article previews some of the advances in cancer immunotherapy that are bound to be hot topics at CHI’s Protein and Antibody Engineering Summit (PEGS), which will take place at the end of the month in Boston.
One advance concerns the use of antibodies to overcome cancer-directed immune suppression. “How the immune system recognizes tumor cells is still a bit of a mystery,” says Gordon Freeman, Ph.D., associate professor of medicine at the Dana-Farber Cancer Institute. “What we do know is that our immune system continuously eliminates tiny tumors in a process called immune editing, until some tumor cells develop the ability to turn off the immune response.”
For the past decade, Dr. Freeman’s lab developed an extensive body of knowledge regarding inhibitory signals conveyed by tumor cells via the programmed death 1 (PD-1) receptor and its ligand PD-L1. Interaction between PD-1 receptor and its ligand deactivates CD8 T cells and inhibits their capacity to kill tumor cells and make cytokines that recruit other immune cells. PD-L1 is expressed in about one-third of solid tumors including a wide variety of types, and its interaction with PD-1 presents an attractive target for therapeutic manipulations.
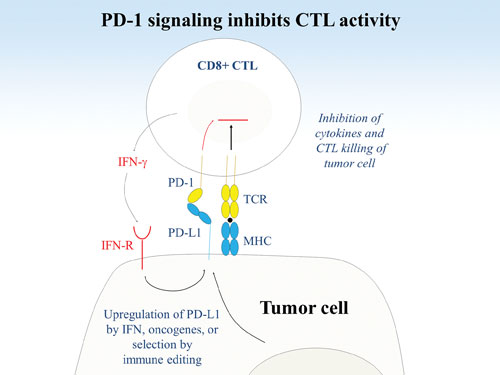
Researchers at the Dana-Farber Cancer Institute are studying immunoregulation via PD-1 and its ligand, PD-L1. (A) PD-L1 can be upregulated on tumor cells by interferons or oncogenic changes. Tumor evolution by immune editing can select for PD-L1 expression to enhance immunoevasion. Engagement of PD-1 on an antigen-specific T cell by PD-L1 on the tumor attenuates the TCR signal, reducing cytokine production and CTL lysis of tumor cells.
Dana-Farber holds 12 issued patents related to the PD-1 pathway, and much of the patent portfolio is nonexclusively licensed to several major pharmaceutical companies. Merck’s fully humanized PD-1 antibody (lambrolizumab) is currently in multiple clinical trials for various solid tumor indications and has received breakthrough therapy designation from the FDA, which puts it on an expedited development path.
“Therapies directed against immune response modulators, such as CTLA-4, were explored before,” notes Dr. Freeman. “Unfortunately, these therapies increased the likelihood of developing an autoimmune response. Anti-PD-1 compounds seem to have considerably milder adverse effects, and therefore are well suited for combination therapies with other immuno- or kinase inhibitors.”
Anti-PD-1 antibodies are infused every several weeks for up to two years. One such antibody, Bristol-Myers Squibb’s Nivolumab, has been evaluated in a recent clinical trial. Nivolumab demonstrated a rapid reduction in kidney tumor volumes, which was followed by almost a half-year of progression-free survival in 67% of study participants.
Dr. Freeman notes that different tumors may employ different immune inhibitory pathways, and some of them may also serve as druggable targets for tumor immunotherapy. He envisions the future of cancer treatment when each individual tumor may be assayed for its specific immunosupressors and oncogenes, and this molecular information will inform the choice of the therapy.
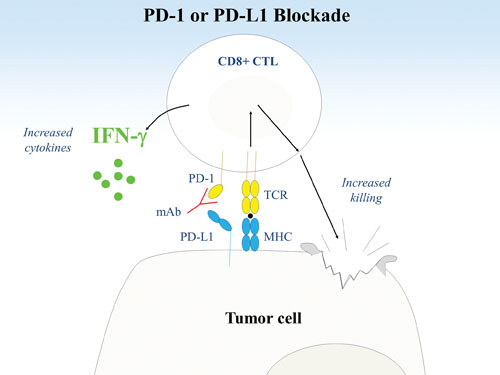
Researchers at the Dana-Farber Cancer Institute are studying immunoregulation via PD-1 and its ligand, PD-L1. (B) Blockade of the PD-1 pathway by PD-1 or PD-L1 mAbs results in a stronger TCR signal with increased T cell cytokine production and killing of tumor cells.
Attacking Cancer-Initiating Stem Cells
Tumor antigen (TA)-specific monoclonal antibodies have become valuable tools in the treatment of cancer. The degree to which TA-specific antibodies are overexpressed by tumor cells in comparison with the normal cells varies depending on the tumor type, but could reach 100-fold.
“We have been developing monoclonal antibodies to TA, which play a role in the biology of tumor cells,” says Soldano Ferrone, M.D., Ph.D., a lecturer on surgery at Massachusetts General Hospital. “Even without conjugation to cytotoxic or radioactive agents, TA-specific monoclonal antibodies can significantly reduce tumor proliferation, migration, and tissue invasion. However, TA-specific monoclonal antibody immunotherapy has limited efficacy. Cancer often comes back.”
Dr. Ferrone postulates that cancer-initiating cells escape immunotherapies and give rise to disease recurrence. In vitro data indicate that, indeed, cancer-initiating cells show limited sensitivity to detrimental effects of TA-specific monoclonal antibodies, even if they are combined with chemotherapeutic agents or radiation.
The team turned to the agents that specifically target aberrant signaling pathways in cancer stem cells. They zeroed in on inhibitors of the Hedgehog signaling pathway, such as Novartis’ LDE-225. Laboratory data with the combination of a Hedgehog inhibitor and one of the unique TA-specific monoclonal antibodies developed in Dr. Ferrone’s laboratory points at a likely clinical efficacy. “If our hypothesis is correct,” continues Dr. Ferrone, “combination of TA-specific monoclonal antibodies and signaling inhibitors could be developed for each type of cancer.,”
Dr. Ferrone has authored the intellectual property covering human monoclonal antibodies developed specifically against two promising TA-specific monoclonal antibodies, a chondroitin sulfate proteoglycan (CPSG-4) and a member of the heat-shock protein family. The latter monoclonal antibody uniquely recognizes an extracellular epitope of this antigen, which is selectively expressed on the membrane of many types of tumor cells, but has very limited expression on normal cells.
The monoclonal antibody is licensed to ImmunGene for development as a part of the company’s antibody-drug conjugates portfolio. Dr. Ferrone applies his strategy to destroy not only differentiated cancer stem cells, but also cancer-initiating cells in head and neck cancer, triple negative breast cancer, pancreatic ductal adenocarcinoma, and chordoma.
This story has been corrected from an earlier version, which misidentified the company to which Dr. Ferrone licensed the antibody as ImmunoGen, not ImmunGene. GEN regrets the error.
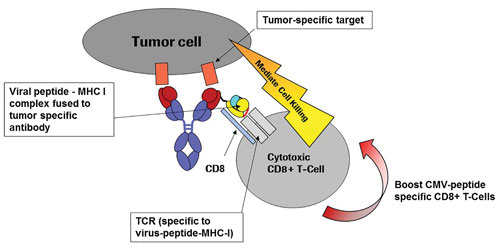
Concept of specific tumor cell lysis developed by Roche: Peptide-MHC class I IgG fusion proteins (pMHCI-IgG) guided via the monoclonal antibody part binding to a tumor-cell-specific surface antigen. The peptide-MHC class I complex (pMHCI) is displayed on the tumor cell surface and attracts preexisting CD8+ effector T cells recognizing the pMHCI complex via their specific T cell receptor (TCR). Recruited CD8+ T cells mediate tumor cell lysis. The pMHC-TCR interaction may also induce the expansion of the specific subpopulation of CD8+ T cells, further enlarging the pool of effector cells.
Genetically Engineered T Cells
“Cancer immunotherapy has already demonstrated substantial clinical benefit,” says Nabil Ahmed, M.D., an associate professor at Baylor College of Medicine’s Center for Cell and Gene Therapy. “We are working on next-generation immunotherapeutics that act as precisely as antibodies yet incorporate advantages offered by the cellular arm of the immune system.”
Dr. Ahmed points out that adoptively transferred immune cells are a customizable living product that offers enhanced penetration of solid tumors. His team uses patients’ own genetically modified T cells carrying chimeric antigen receptors (CARs). CARs are synthetic molecules that consist of an extracellular tumor antigen-binding domain and an intracellular cytoplasmic signaling domain, which enables T-cell activation.
Three of Dr. Ahmed’s clinical pilot studies received FDA approval to use T cells from glioblastoma and solid tumor patients modified with CARS targeting for human epidermal growth factor receptor 2 (HER2). In preclinical studies, it was shown that T cells grafted with a HER2-specific CAR readily kill primary HER2-positive tumor cells and cancer stem cells. Also, it has been shown that these T cells eliminate human tumors in animal models. These cells readily travel into the tumor tissue and do not abide by human leukocyte antigen restrictions.
Dr. Ahmed’s team has worked closely with the FDA to ensure that their cellular therapy product meets the agency’s requirements for limited circulation time. However, the short-lived efficacy could be further enhanced by introducing co-stimulatory components based on latent viruses, such as cytomegalovirus.
“Our therapy approach demonstrated promising early results, such as a very favorable safety profile and objective tumor responses,” continues Dr. Ahmed. “However, some cells within the same tumor may not express the target antigen, and many down-regulate it in response to therapy. Our new generation of broad-spectrum products is designed to target two or more tumor antigens simultaneously.”
This sophisticated approach involves T cells’ expression of multiple antigen-specific CARs or employing tandem CARs with two distinct antigen recognition domains on a single transgenic receptor. The team used computational modeling methods to make structure predictions and to analyze and rationally design the tandem CARs. The proof-of-concept molecule effectively recognized each tumor antigen distinctly and exhibited maximum functionality upon encounter of both simultaneously. Dr. Ahmed plans to explore whether tandem CARs will enhance tumor control in patients with glioblastoma and sarcomas.
Harnessing the Antiviral Response
Roche’s pharma research and early development (pRED) program harnessed the power of the adaptive immune response to cytomegalovirus (CMV) to efficiently eradicate cancer cells. CMV is a powerful infectious agent and is continuously cleared out by the immune system. As many as 5–10% of all cytolytic T cells respond to CMV epitopes—a figure higher than that seen with any other human virus.
The specific T-cell response against CMV is based on their memory function. According to Hendrik Knoetgen, Ph.D., head of lead identification at Roche Diagnostics, “Among every 100 adults in the United States, 50–80 are infected with CMV by the time they are 40 years old. Most of them do not experience any symptoms because of the high numbers of circulating memory T cells. We use CMV peptide-major histocompatibility complexes (pMHCs) to attract these T cells to cancer cells.”
In theory, the CMV-MHC class I complexes, if presented on the surface of cancer cells, would be able to recruit cytotoxic T cells, which in turn would induce targeted cell lysis. However, expression of the immune complexes has proven to be exceedingly difficult. Roche’s creative strategy successfully delivers MHC-CMV peptide complexes to the surface of tumor cells via an antibody carrier.
The pRED team created single-chain peptide-MHC constructs fused to full-length immunoglobulins, such as immunoglobulin G (IgG), and it generated fusion proteins of quality and purity comparable with typical IgG production. The antibodies are directed against specific tumor antigens. When bound to the cancer cells, the antibodies bring the CMV peptide-MHC complex with them. Cytotoxic CD 8+ T cells isolated from the blood samples of healthy CMV-positive donors eliminated all melanoma cells within 42 hours after incubation with the IgG-CMV-MHC fusion protein.
Dr. Knoetgen explains that one T cell can kill multiple target cancer cells. “The only limitation to this technology,” he explains, “is that MHC molecules are highly polymorphic. Our product has to match the native human histocompatibility complex of the given patient. Initially, we focus on the most commonly occurring HLA types, but in the future our methodology could be applied to generate personalized medicines using a patient’s own genetic background.” The team is completing evaluation of the technology in animal models and finalizing the choice of the target tumor antigens.
Host-Protective Adaptive Immunity
“This is a very exciting time in the cancer immunotherapy field,” says Louis M. Weiner, M.D., director of Georgetown University’s Lombardi Comprehensive Cancer Center. “Monocolonal antibodies for tumor therapy are well established as effective therapeutic agents. Most research in the past focused on the ability of these antibodies to affect signaling pathways in target tumor cells. A new body of knowledge emerges to strongly suggests that TA-antibodies also elicit tumor-antigen specific immune responses.”
Dr. Weiner is exploring how antibody-dependent cellular toxicity (ADCC) could be used to liberate preexisting host immune responses to tumor cells. ADCC is well established as a relevant antitumor mechanism of action, but its role in stimulating a vaccine-like immune reaction is less well understood.
To learn more about cellular immune responses triggered by ADCC, Dr. Weiner’s team created a new transgenic mouse model. The mouse carries a mutated version of HER2, a well-known human tumor antigen. This inactive protein is ubiquitously expressed in normal tissues and thus became a self-antigen. After mice were dosed with melanoma cells, transtuzumab (Herceptin, a specific anti-HER2 antibody) was used to promote killing of tumor cells.
“We further stimulated the immune response by adding a potent synthetic agonist of T cells, which acts via Toll-like receptors,” continues Dr. Weiner. Mice treated with the combination therapy not only survived longer, but also rejected re-challenge with the same melanoma cells. Dr. Weiner views it as an evidence of the induction of host-protective adaptive immunity. The key, he says, is to amplify this response to make it clinically relevant.
His team deployed synthetic lethal screens using small interfering RNAs to identify regulators of tumor cell-based resistance in the signaling network of EGFR, another well-established tumor antigen. This comprehensive study revealed no particular Achilles heel that could be exploited to reverse resistance to EGFR-targeted therapeutics, underscoring that the fitness of cancer cells is determined by the robustness of their related signaling networks.
Nevertheless, a few potential targets have emerged. For example, inhibition of ABL1, a gene that when fused to the BCR gene plays a critical role in chronic myelogenous leukemia, enhanced ADCC and promoted adaptive immunity.
Based on these data, Dr. Weiner and colleagues have initiated a clinical trial employing cetuximab (Erbitux) and nilotinib in patients with advanced head and neck cancer. The study will test the hypothesis that this combination will improve the initial efficacy of cetuximab therapy while eliciting a lasting host tumor-directed adaptive immune response that contributes to increased patient survival.



