September 1, 2017 (Vol. 37, No. 15)
Proteases in Some Cancer Cells Have the Ability to Break Antibodies by Taking Them Off Their Hinges
There is a critical need to think about new paradigms and new ways of approaching therapy in oncology, according to Edward F. Patz Jr., M.D., the James and Alice Chen Professor of Radiology at Duke University and CEO, GRID Therapeutics.
“We have not, despite the fact that we’ve learned a lot, had a dramatic impact on outcomes,” he emphasizes.
Antibodies—immune-reactive molecules that play key roles in engaging the immune system—are the cornerstones of emerging immunotherapies. “However, tumor resistance to antibody therapy has become a major problem,” informs Zhiqiang An, Ph.D., professor and Robert A. Welch Distinguished University Chair in Chemistry, University of Texas Health Science Center at Houston. Tumors can develop immune-evasion mechanisms that disable antibody-mediated effector functions, and strategies are needed to counteract evasion.
On the other hand, effectorless antibodies may be novel therapeutic routes for neurodegenerative diseases associated with chronic brain inflammation, enlightens James A. Ernst, Ph.D., senior scientist, Genentech.
Different approaches for developing therapeutic antibodies were showcased at the PEGS Engineering Antibodies conference held in May 2017 in Boston. Herein, we highlight innovative technologies from the presentations of Drs. Patz, An, and Ernst; Patrick S. Daugherty, Ph.D., CSO, SerImmune, and adjunct professor, University of California, Santa Barbara; and Bill Harriman, Ph.D., CSO, Crystal Bioscience.
Powered by Divergent Evolution
Antibody development starts with your target antigen, which in the therapeutic antibody space is typically a human protein. If you opt for a traditional hybridoma, a mouse will usually make at least some antibodies against your human protein. However, “if your human target is very similar to the murine ortholog, the mouse may regard that target as a self-protein and not make antibodies against it,” warns Dr. Harriman. “Once you get above 95% amino acid identity, it can be very difficult to raise antibodies in mice.”
Crystal Bioscience’s transgenic HuMab Chicken platform exploits divergent evolution between birds and mammals to make antibodies against highly conserved mammalian targets such as brain-derived neurotropic factor (BDNF), which is 97% identical between human and mouse, but only 91% between human and chicken. “That level of divergence can make the critical difference in whether you get a titer when you immunize your animals and whether you can go on to recover monoclonals,” says Dr. Harriman.
A good fusion partner hasn’t been found for chickens, so Crystal Bioscience developed a unique single B-lymphocyte cloning strategy that avoids the hybridoma step altogether. “GEM (gel-encapsulated microenvironment) screening allows you to take hundreds of millions of individual lymphocytes from an immunized animal and put them in little droplets containing reporters,” explains Dr. Harriman. Reporters are antigen-coated beads and/or cells presenting native antigen on their surfaces that can be used to directly screen for individual lymphocytes of interest, which are recovered and transformed into fully human recombinant antibodies.
Chickens, powered by divergent evolution, can produce antibodies against a wide range of conserved mammalian epitopes that mice may regard as self. “Species–cross-reactive antibodies are highly desirable for antibody-drug development,” informs Dr. Harriman. When you raise your therapeutic in chicken and produce a cross-reactive antibody, you can potentially start your preclinical animal model with the same antibody you’ll use for clinical studies. “That’s a big advantage for our pharma partners,” concludes Dr. Harriman.
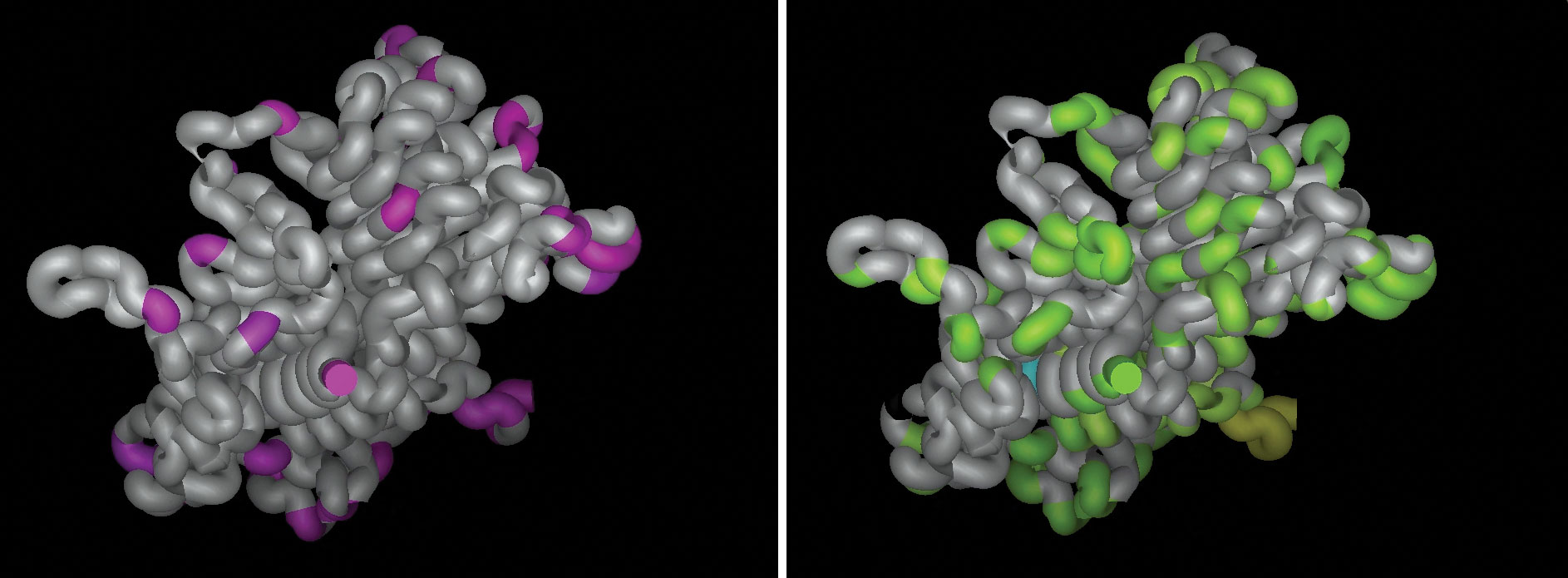
To expedite antibody discovery, Crystal Biosciences exploits immune systems of animals that are evolutionarily distant from humans. For example, the company has identified potentially useful epitopes by mapping the sequence differences between HER2 extracellular domains in different species—specifically, mice and humans (left, purple), and chickens and humans (right, green). Regions of human HER2 highlighted in green but not pink represent epitopes that may be uniquely recognized by a chicken immune response. Chicken hosts have greater opportunities than mice to see mammalian epitopes as foreign and mount robust and diverse immune responses.
Mapping Targets of Antibody Repertoires
Immunological memory is like a hard drive that records the millions of antigens to which humans are exposed. “SerImmune is building the capability to read back all that information embedded in the immune hard drive,” says Dr. Daugherty.
SerImmune’s immune-mapping technology and antibody repertoire database platforms characterize human antibody repertoires at the level of their epitope-binding specificities to identify the target antigens of many antibodies in parallel.
“We use a large peptide library that has about 5–10 billion members, which is followed by next-generation sequencing (NGS),” explains Dr. Daugherty.
NGS looks at millions to billions of peptides that bind to the antibodies and mimic the antigens. Motif discovery informatics tools identify thousands of features in the NGS peptide sequence data, which correspond to the epitopes characterized by the antibodies. Subsets of these features associate with a disease phenotype such as cancer, infectious or autoimmune disease.
A proof-of-principle study identified an eight-residue consensus wheat gliadin antigen motif that bound to antibodies in sera from a celiac disease cohort and not in the control group. Although antigen specificities were identical in both groups, individuals’ anti-gliadin antibody sequences were different. The identification of public antigens like the gliadin motif is possible because SerImmune focuses on the antigens as compared to the gene sequences of antibodies. “By building a database of thousands of tested specimens that can be analyzed for hundreds of different antigens, we expect we will be able to identify new associations between environmental factors and human disease. We are very interested in working with partners who have a need to identify disease-associated antigens,” concludes Dr. Daugherty.
Outwitting Hinge Cleavage
“The hinge that links Fc effector and Fab antibody-binding regions is like an Achilles’ [heel] for the antibody,” remarks Dr. An. Hinge flexibility is required for antibodies to interact with Fc receptors to mediate antibody-dependent effector functions, but also makes antibodies vulnerable to protease attacks.
Bacterial cells secrete proteases that cleave the hinge. “Once one strand of the hinge region is cleaved, the efficacy of the effector function is severely compromised,” emphasizes Dr. An. “Cancer cells, like bacteria, are also pathogenic cells. My lab, in collaboration with Janssen Pharmaceuticals, developed technology to show that cancer cells in the tumor microenvironment secrete proteases, such as matrix metalloproteases, which also cleave the hinge region.”
Dr. An evaluated the efficacy of Herceptin (trastuzumab) IgG1 in an animal model with tumor-overexpressed proteases, which demonstrated that hinge cleavage is a mechanism tumors use to evade antibody (humoral) immunity. Trastuzumab exerts its therapeutic effects through Fab-mediated HER2 signaling inhibition and Fc-mediated effector functions. “When trastuzumab gets cleaved, it only gives you partial efficacy,” informs Dr. An.
The team outwitted the proteases by engineering a proteolytic cleavage-resistant form of trastuzumab. “This mutated trastuzumab gave 100% tumor inhibition,” Dr. An points out. In another approach, the team developed a rescue antibody that recognizes a neoepitope exposed during cleavage and has a mutated Fc that engages the immune system. “This antibody restored full efficacy of cleaved trastuzumab,” emphasizes Dr. An.
“Humans produce autoantibodies targeting oncogenes such as HER2, which are part of human immune surveillance,” enlightens Dr. An. “Autoantibodies help us prevent tumors from happening in the first place, but are also subject to cleavage inactivation.” Cleavage-resistant or rescue antibodies potentially have broad applications for mitigating antibody drug resistance or overcoming tumor evasion of autoantibodies.
Effectorless Interception of Extracellular Tau
“Tau is a very attractive target for Alzheimer’s disease because the accumulation of tau pathology is intimately correlated with cognitive decline,” says Dr. Ernst. Tau is normally an intracellular soluble protein and spreading is comprised of extracellular releases. One cell releases tau and another cell takes it up in a prion-like fashion and converts normal tau into pathological tau (insoluble neurofibrillary tangles).
“Our antibody targets tau in the extracellular environment and sequesters it. The antibody blocks tau pathology from spreading throughout the brains of mouse models,” says Dr. Ernst. “When our antibody binds tau in the extracellular environment, we think it intercepts tau and disables its ability to be taken up by another neuron,” remarks Gai Ayalon, Ph.D., scientist and tau project team lead, Genentech.
Microglia drive immune responses in the brain that are recruited by antibody-Fc effector regions. Genentech scientists were concerned that any benefits derived from utilizing immune effector function to clear tau with an antibody could aggravate pre-existing neuroinflammation. They asked, “is effector function needed to effectively target tau?”
The scientists looked at several different metrics of tau levels in the brain of a tau disease model mouse. “We saw the same outcome with tau antibodies that were effector positive with full effector function or effector attenuated (effectorless),” notes Dr. Ernst. Hence, normal effector function wasn’t needed to achieve the desired therapeutic outcome in the mouse. Genentech currently has an anti-tau antibody in human clinical trials.
Autoantibody Overcomes Tumor Immune Evasion
“There are cohorts of cancer patients who are pathologically given the diagnosis of cancer, but do exceptionally well,” says Dr. Patz. His lab discovered an autoantibody to complement factor H (CFH), a high abundance serum protein, found in early-stage non-metastatic patients who did exceptionally well. The autoantibody was not found in patients with late-stage disease or those with early-stage disease who developed metastasis.
The CFH autoantibodies from all the patients who made them mapped exactly to the same eight amino acid domain, recognizing a cryptic epitope that appears to be exposed only on conformationally altered tumor-bound CFH. The patients with exceptional outcomes may have developed the autoantibody to overcome tumor evasion of humoral immunity when CFH bound to tumor cells, allowing protection against complement lysis.
We developed a new strategy to “make the first completely human-derived antibodies for oncology,” says Dr. Patz. This involved pulling out B cells from patients making the CFH autoantibody, and sorting out single B cells by fluorescence-activated cell sorting (FACs) against the specific epitope. The immunoglobulin genes of the B cells were sequenced and the sequences transfected into mammalian cells to make recombinant antibodies. These CFH antibodies, just like the patients’ autoantibodies, bind to tumor-bound CFH and not to normal CFH circulating in the blood.
Tumor-bound CFH is a mechanism of rituximab resistance in patients with CLL. “The tumor cells use tumor-bound CFH to protect themselves against complement lysis,” says Dr. Patz. The CFH recombinant antibody can block the function of tumor-bound CFH, allowing rituximab-induced complement lysis. GRID Therapeutics has licensed the CFH antibody from Duke. “We’ve begun manufacturing and we’re moving the antibody forward to a clinical trial,” concludes Dr. Patz.



