July 1, 2008 (Vol. 28, No. 13)
Clinical Utility of Pharmacogenomics and Genetic Testing Remains an Issue
Through rapid advances in research and clinical applications, molecular diagnostics has grown from a genomic and proteomic analysis tool into a powerful new conduit for healthcare practitioners to deliver improved patient outcomes.
When it comes to identifying pathogens, whether it be bacterial or viral, molecular diagnostics is superior to traditional methods, allowing for direct detection with greater sensitivity and specificity. Molecular diagnostics used as companion diagnostics serve admirably to direct highly targeted therapies. Genetic testing and pharmacogenomics tests, however, are more difficult to justify in terms of clinical utility.
With skepticism abounding, there needs to be evidence that medical intervention following such tests actually increases clinical benefits. Studies are currently under way to collect such evidence, although there are already many who believe in the potential of genetic testing and pharmacogenomics testing.
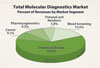
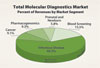
Nonetheless, industry participants in the molecular diagnostics market cannot rely on early adopters alone. Growth in molecular HIV, HBV/HCV, CT/NG, and HPV testing, all of which make up the bulk of the molecular diagnostics market, is slowing. Given the restraints in healthcare spending, the development of new tests and systems that can truly demonstrate their clinical value is critical for widespread adoption.
Adoption of Tests
Molecular diagnostic tests originate from several different manufacturers. Furthermore, these tests run on different systems that end-users have to install should they wish to perform them. The market is fragmented, and there is no single vendor that can provide a comprehensive suite of products. Thus, only clinical laboratories with substantial resources for implementation can position themselves to provide molecular diagnostics to the medical community.
This is further compounded by the fact that most molecular diagnostic tests are highly complex and limited to those that meet the requirements set forth by the CLIA. But given the small test menu on each system, it is primarily the volume of tests that warrants adoption, and again, this fits the role of the select few that are positioned to provide molecular diagnostics. Take into consideration the limited number of clinicians available to perform such testing and we see how challenging it is to increase adoption.
The growing concern about hospital-acquired infections, namely MRSA, is expected to prompt more hospitals to embrace molecular diagnostics for screening patients.
The use of molecular diagnostics with real-time PCR-based tests can greatly expedite the testing process and deliver results within hours for rapid medical intervention. Hospitals have to determine the best system for their needs. With continued political debate about hospital-acquired infections, MRSA testing represents a prime opportunity for manufacturers to penetrate into more hospitals.
Genetic and pharmacogenomics testing, however, appear to be more suited for reference laboratories and large medical research institutions.
In addition to skilled laboratory technicians, experts are needed to comprehend the significance of the test results and educate both patients and physicians. Naturally, the limitations of genetic and pharmacogenomics testing have raised doubts. Until more data is collected to measure the clinical utility of these tests, the market for genetic and pharmacogenomics testing will continue to teeter on physicians’ mixed perceptions.
With the limited number of tests and systems offered by individual manufacturers, the ideal suite of products has yet to be developed and that is reflected in the lack of adoption among the majority of healthcare institutions. Instead, orders for molecular diagnostic tests are being consolidated into select clinical laboratories that have the resources to perform them.
In order to make molecular diagnostics more accessible and allow for overall market growth, several issues need to be addressed. Innovations in molecular diagnostics continue to advance the state of medicine, but in an increasingly cost-conscious healthcare environment with payers having more and more influence over treatment decisions, manufacturers must consider the clinical value of their products and services.
Kevin Leong is a research analyst in the drug discovery technologies and clinical diagnostics group of Frost & Sullivan. Email: [email protected].