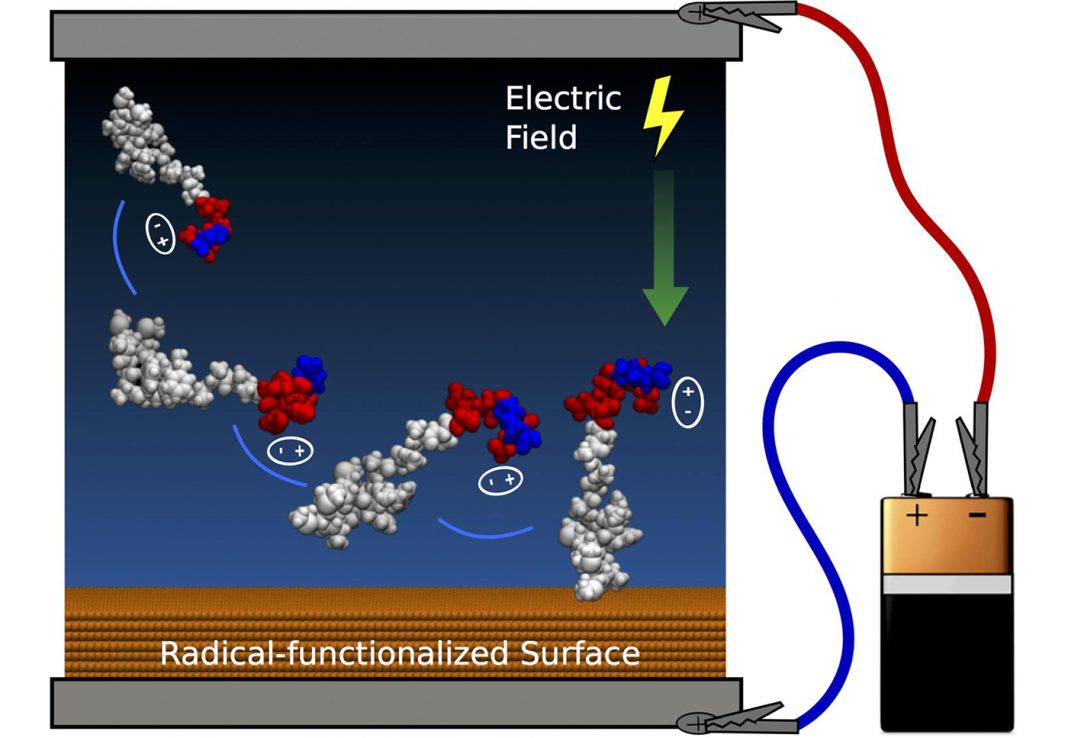
Scientists at the University of Sydney’s Applied Plasma Physics and Surface Engineering Laboratory say they have developed effective techniques to guide and attach peptides to surfaces. Computer simulations and experiments demonstrated control of both peptide orientation and surface concentration, which can be achieved by applying an electric field like that delivered by a small household-sized battery, they add.
The researchers, who published their study (“Electric Fields Control the Orientation of Peptides Irreversibly Immobilized on Radical-Functionalized Surfaces”) in Nature Communications, believe their work can lead to new types of implantable devices that provide biological signals to surrounding tissue for better integration with the body and reduced risk of infection.
“Surface functionalization of an implantable device with bioactive molecules can overcome adverse biological responses by promoting specific local tissue integration. Bioactive peptides have advantages over larger protein molecules due to their robustness and sterilizability. Their relatively small size presents opportunities to control the peptide orientation on approach to a surface to achieve favourable presentation of bioactive motifs,” write the investigators.
“Here we demonstrate control of the orientation of surface-bound peptides by tuning electric fields at the surface during immobilization. Guided by computational simulations, a peptide with a linear conformation in solution is designed. Electric fields are used to control the peptide approach towards a radical-functionalized surface. Spontaneous, irreversible immobilization is achieved when the peptide makes contact with the surface. Our findings show that control of both peptide orientation and surface concentration is achieved simply by varying the solution pH or by applying an electric field as delivered by a small battery.”
“The holy grail is a surface that interacts seamlessly and naturally with host tissue through biomolecular signaling,” said Marcela Bilek, Ph.D., who is a member of the University of Sydney Nano Institute and the Charles Perkins Center. “Although proteins have successfully been used in a number of applications, they don't always survive harsh sterilization treatments and [can] introduce the risk of pathogen transfer due to their production in microorganisms.”
According to Lewis Martin, a Ph.D. candidate in the School of Physics, the team has focused on the use of peptides that, when strategically designed, can recapitulate the function of the protein. Behnam Akhavan, Ph.D., the School of Aerospace, Mechanical and Mechatronic Engineering and the School of Physics, added that with industry support and funding for clinical trials, improved implants could be available to patients within five years.
“The application of our approach ranges from bone implants to cardiovascular stents and artificial blood vessels,” Dr Akhavan said. “For the bone implantable devices, for example, such modern biocompatible surfaces will directly benefit patients suffering from bone fracture, osteoporosis, and bone cancer.”