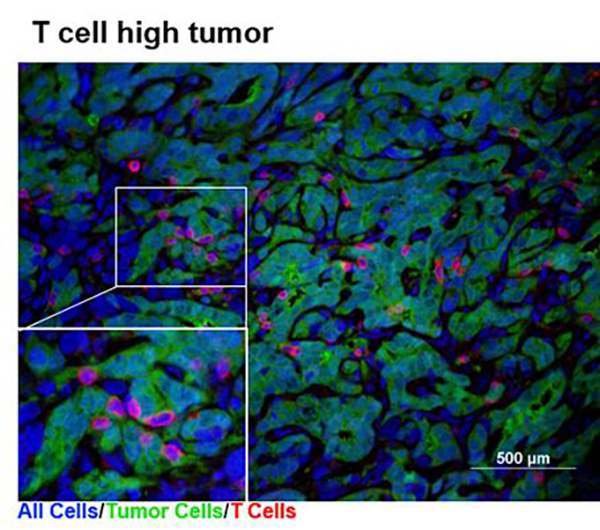
Pancreatic cancer, which was predicted to become the second leading cause of cancer death as early as 2015, eludes treatment for several reasons, not the least of which is tumor heterogeneity. For example, pancreatic tumors may run “hot” or “cold”—influencing how they respond to immunotherapy. Hot tumors are filled with T cells. Cold tumors have fewer T cells and are often considered to be less sensitive to immunotherapy.
But how does a pancreatic tumor become hot or cold in the first place? And can a pancreatic tumor’s “temperature” be controlled to enhance immunotherapy?
To answer these questions, scientists based at Penn Medicine's Abramson Cancer Center (ACC) probed tumor heterogeneity, the ways tumor cells manifest diversity with respect to movement, replication, metastasis, and treatment response. After experimenting with a library of pancreatic cancer cell clones by implanting them in immunocompetent mice, the scientists determined that tumor-cell-intrinsic factors shape the tumor immune microenvironment and influence the outcome of immunotherapy.
Detailed results appeared June 26 in the journal Immunity, in an article entitled “Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy.” According to this article, cell lines implanted in normal mice with a working immune system grew into tumors that fell into the hot and cold categories, with cold tumors being the dominant type. In addition, the article indicates that whether a tumor was hot or cold determined whether it would respond to immunotherapy.
“Transcriptomic and epigenetic analyses revealed tumor-cell-intrinsic production of the chemokine CXCL1 as a determinant of the non-T-cell-inflamed microenvironment,” wrote the article’s authors, “and ablation of CXCL1 promoted T cell infiltration and sensitivity to a combination immunotherapy regimen.”
Half of the mice with hot tumors experienced tumor regressions after treatment with a checkpoint blockade drug, an effect that was enhanced with the addition of either an anti-CD40 agonist, combined chemotherapy, or both. Of the 26 mice bearing hot tumors and treated with a combination of chemo- and immunotherapy called GAFCP, 20 survived to more than six months, suggesting a durable response to the therapy. By contrast, none of the mice with cold tumors cleared their cancer following this therapy.
To understand the molecular basis of this phenomenon, the team searched for factors released by cold tumors that could attract myeloid cells. They found that cold tumor cells make a compound called CXCL1, which signals myeloid cells to enter tumors and T cells to stay away, which eventually results in insensitivity to immunotherapy. Conversely, knocking out CXCL1 in cold tumors promoted T-cell infiltration and sensitivity to immunotherapy.
Recent studies from Penn Medicine and other institutions have suggested that the degree to which T cells are attracted to a tumor is regulated by genes specific to that tumor. “There is no disputing that targeting immune cells has led to promising outcomes for many cancer patients, but not every person responds to these types of treatments,” remarked Ben Stanger, M.D., Ph.D., the senior author of the current study and a professor of gastroenterology and cell and developmental biology in the Perelman School of Medicine at the University of Pennsylvania. “Every tumor is different, so we're investigating how to use the underlying biology of tumor cells to successfully treat more cancer patients.”
The cell lines that the team generated mimic a spectrum of pancreatic tumor features, including the types of immune cells they contain. In the future, these tumor cell lines could help to further identify and optimize therapies for specific subsets of patients with various states of tumor heterogeneity.