April 15, 2017 (Vol. 37, No. 8)
RenovaCare’s CellMist System Promises to Accelerate the Healing of Burns and Wounds
A technology for spraying a patient’s own stem cells onto severe burns is helping patients to leave the hospital within days of being admitted, rather than the weeks typical for standard-of-care grafting techniques. Developed by RenovaCare, this autologous skin replacement technology—the first of its kind—results in scar-free healing.
Before stem cells can be sprayed, they need to be isolated from a skin sample and suspended in liquid. Nevertheless, the whole process—the isolation, suspension, and spraying of stem cells—takes as little as 90 to 120 minutes.
The process begins with a small sample (about 2 inches by 2 inches) of healthy skin. “We isolate the cells by removing the surrounding tissue,” says Thomas Bold, RenovaCare’s president and CEO. The cells are suspended in liquid, resulting in a product called the CellMist™ Solution, which is inserted, by way of syringe, into the SkinGun™, a patented device that can be used to spray the CellMist Solution into a wound gently and evenly.
The cells aren’t heavily manipulated, and no cultivation step is necessary before they are sprayed into wounds. “Our process results in skin that looks and functions like the patient’s own skin—which it is,” asserts Bold. “With mesh grafting, the current standard of care, you often don’t achieve good clinical resolution.”
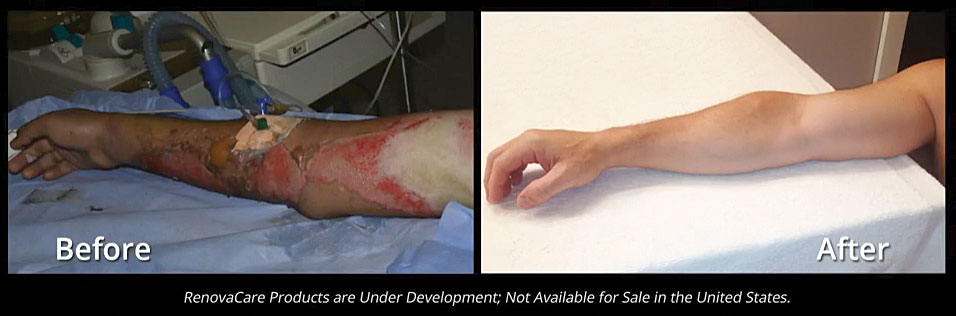
These images depict the recovery of a severely burned state trooper who was treated with the RenovaCare CellMist System, which comprises the company’s CellMist Solution and SkinGun.
The Cell Gun
When RenovaCare first began spraying stem cells, the company wasn’t satisfied with their viability, Bold recalls. The high pressure created by pump-spray systems ruptured many of the fragile stem cells. A gentler approach was needed.
“We developed a technology where the cells are guided through the syringe and are picked up by an air stream,” Bold details. “The needle is inside a tube that’s pushing air, so the cells are nebulized outside.” This gentle administration method enables a cell survival rate that exceeds 97%.
Tests conducted at Berlin-Brandenburg Center for Regenerative Therapies, a research center at Charité-Universitätsmedizin Berlin, reported the SkinGun sprays 200 times more droplets of CellMist Solution than does conventional needle and syringe deposition, resulting in the deposition of 20,000 cells versus 91. The dispersal pattern is even, and growth rates are comparable to pipetting, which is considered the gold standard for cell deposition.
Developing the SkinGun as a viable device took a couple of years. Patents for that work were issued in Germany. The company’s first U.S. patent was issued at the beginning of 2017 for a delivery system that is more advanced, smaller, and physician-friendly. This new patent reinforces the company’s German patents and extends protection an additional 30 months, to beyond the year 2035.
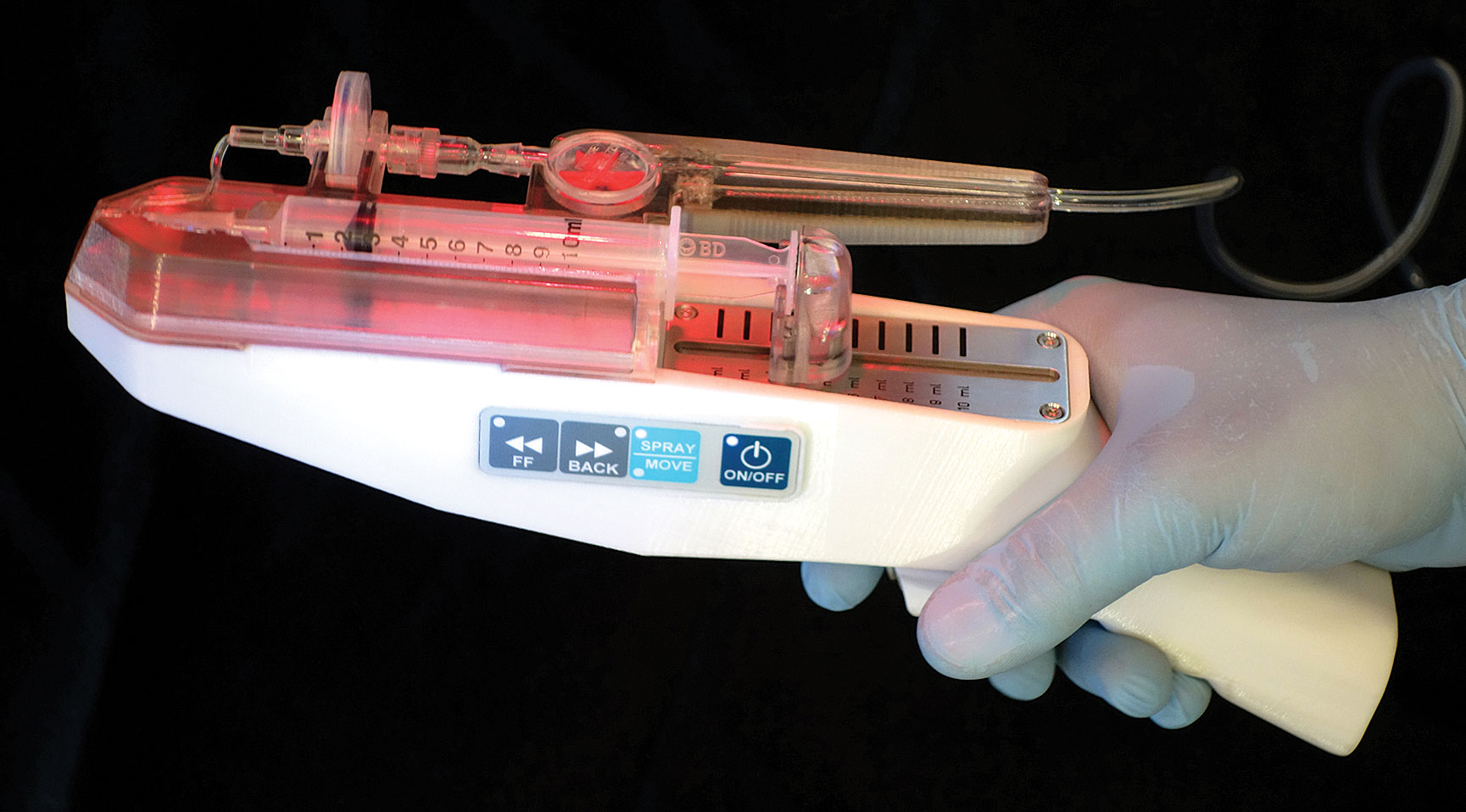
The RenovaCare SkinGun gently sprays a patient’s own stem cells onto severe burns for rapid, scar-free healing. The device achieves a high rate of cell viability (97.3%), which is essential to regenerating skin for burns and chronic wounds.
Accelerated Healing
The benefits to patients include the need to harvest less healthy skin, and dramatically faster wound healing. With RenovaCare’s CellMist and SkinGun, which together constitute the CellMist System, one square centimeter of healthy skin can be used to treat 100 square centimeters of burned skin. With traditional skin grafting techniques, that square centimeter could only treat about six square centimeters.
Healing is accelerated because the stem cells are sprayed into the wound, stimulating islands of regeneration throughout the wound. “Wounds normally heal from the edges toward the middle,” Bold points out. RenovaCare’s method triggers healing all over the wound.
RenovaCare is focused on severe second-degree burns where dermal structures remain. The CellMist System therapy also may be valuable as an adjunct therapy in dermal replacement, Bold observes. Other possibilities include treatments for skin pigmentation disorders (such as vitiligo) and scarring (including acne scarring) as well as cosmetic applications. The company also is developing sprays for wound care as well as the delivery of irrigation fluids.
This technology has the potential to replace traditional skin grafts. If that happens, hospital stays might be reduced by many weeks. Also, the risk of infections and other complications might be reduced, along with the pain and expense of harvesting healthy skin to apply to injured sites.
Origin
RenovaCare was formed about four years ago and was based on work that was undertaken when Bold was CEO at StemCell Systems. “We were working with a hospital in Berlin,” he recalls. “Its burn center wasn’t satisfied with available therapies, so we were supposed to develop a multilayer skin product.”
The bioreactor technology at the time allowed the team to develop layers of a flat film that would migrate into the wounds. That approach didn’t deliver optimal results, “so we developed the idea of using the wound itself as a bioreactor,” Bold explains. Eventually, StemCell Systems and RenovaCare collaborated to advance the CellMist Solution and SkinGun technologies.
After receiving promising clinical results, RenovaCare is focusing on the business and regulatory aspects needed to eventually commercialize its product. That includes refining its regulatory strategy in preparation for eventual FDA filings. “We’re talking with the FDA, and we expect to begin a new study soon,” Bold tells GEN. Details of that study are not yet public. The company’s staff has been bolstered with the addition of Roger Esteban-Vives, Ph.D., a world-renowned expert on stem cell isolation, as director of cell sciences.
“We’re putting [the next phase of] the business model together and raising funds,” Bold reports. There are no plans to out-license the technology. Wound care represents a $45 billion market in the U.S. alone.
RenovaCare
Location: 430 Park Avenue, Suite 702, New York, NY 10022
Phone: (888) 398-0202
Website: renovacareinc.com
Principal: Thomas Bold, President and CEO
Number of Employees: 10
Focus: RenovaCare has developed spray-on stem cells for use in wound care and other skin conditions. The technology is approved for investigational clinical use in the U.S. and Europe.



