July 1, 2013 (Vol. 33, No. 13)
Singulex Screens Clinical Samples in Search of Biomarkers at the Femtogram Level
The human proteome counts among its ranks hundreds of thousands of unique members, each hinting at a patient’s health status in some way. Scientists have validated many of these proteins as biomarkers, pointing to their diagnostic and prognostic potential.
Elevated troponin, for example, has historically been used as a marker for cardiac arrest in the emergency room. That asymptomatic individuals with elevated troponin levels have increased risk of a cardiac event speaks to the power of the protein as a disease predictor.
Singulex seeks to harness the predictive power of proteins like troponin with its fully automated digital immunoassay system capable of detecting single molecules to the femtogram level. Dubbed Erenna®, the product has been used in large pharmaceutical R&D labs, at academic institutions, and by CROs alike. Singulex customers are using the platform to evaluate therapeutic safety and efficacy, as well as pharmacokinetics and pharmacodynamics, among other things.
Erenna includes a confocal optical platform and background-reduction methodology that enables the quantification of low-abundance biomarkers and extends the dynamic range to over 4 logs, offering improved sensitivity and specificity over existing ELISAs or electrochemiluminescent-based immunoassays, the firm says.
“That’s really a key differentiator which enables us to precisely and confidently monitor very low volume biomarkers over time, using low sample volumes, from discovery to clinical applications,” Singulex CEO Guido Baechler tells GEN.
Launch of this system—and the resulting life science business—helped Singulex take off, priming its natural foray into the clinical realm. “Our life science business was our initial revenue-generating business that enabled us to discover disease applications of unique biomarkers that we are offering today in our clinical lab,” he says.
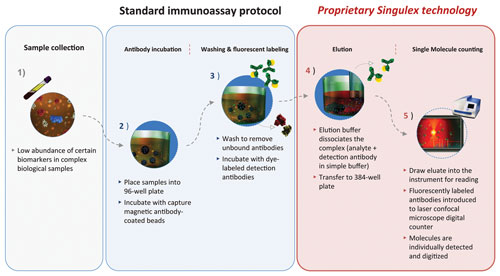
Singulex couples its digital single-molecule counting (SMC) technology with microparticle-based immunoassays to provide higher sensitivity and broader dynamic range over traditional immunoassay platforms.
Diagnostic Monitoring Device
In 2010, the firm began processing customer blood samples through its advanced cardiovascular diagnostic monitoring service. Singulex analyzed more than 170,000 blood samples in its CAP-certified and CLIA-licensed California lab in 2012. To date the company has processed more than 3 million clinical tests, Baechler says, noting that additional diagnostic services are coming down the pike.
Because the technology can be used to detect low-abundance biomarkers in healthy populations, it improves the prognostic and diagnostic power of established biomarkers associated with increased risk for developing cardiovascular diseases, such as heart failure, notes Baechler. “Our technology can improve patient care while reducing healthcare costs,” he suggests.
Baechler points to case studies in which ostensibly healthy individuals, who presented with elevated cardiac troponin and inflammation biomarker levels, were subsequently referred to specialists who, with further monitoring, identified significant risk of a cardiac event.
“We see this quite frequently and that’s really a driver of what we’re doing today,” Baechler says.
“Combining cardiac troponin with cytokines and other inflammatory markers and pairing it with advanced lipid tests, we are able to provide a full panel to physicians to identify patients at risk in a chronic setting for a primary or secondary cardiac event so they can individualize treatment strategies and monitor the effectiveness of therapies over time.”
The company is now working to develop a next-generation, fully automated benchtop immunoassay analyzer that can run up to 15 different tests on a single sample with a turnaround time similar to that of standard platforms, around 20 to 30 minutes.
“This system will allow us to distribute our immunoassay technology globally to hospitals and reference labs,” Baechler says.
At the same time, he adds, the company’s digital immunoassay technology is “continuing to power discovery and development of innovative diagnostics and treatments for chronic diseases beyond cardiovascular disorders,” such as Alzheimer’s, arthritis, and diabetes.
“The ability to measure biomarker concentrations at healthy levels—not possible previously with other technologies—and to monitor small changes in concentrations over time has expanded the clinical utility of proven and emerging biomarkers, thus providing researchers with new insights into disease biology, drug safety, and efficacy,” Baechler says.
Singulex
Location:
1701 Harbor Bay Parkway, Suite 200, Alameda, CA 94502
Phone: (510) 995-9000
Website: www.singulex.com
Principal:
Guido Baechler, CEO
Number of Employees: 247
Focus:
Singulex detects low-abundance biomarkers for diagnostic and prognostic applications in cardiovascular and other diseases.



