May 15, 2011 (Vol. 31, No. 10)
As Speedy Workflows Become Commonplace, Focus Shifts to Highly Reproducible Results
Being tool builders is part of what separates humans from the rest of the animal kingdom. It is only natural that, as scientists, we are drawn toward that which is new, fast, ultra, and novel. Perfinity Biosciences created a system that automates multiple sample-preparation workflows, performing them in a fraction of the time it takes to perform the same steps using traditional methods. Recently, Perfinity had to take a step back when a customer called a time-out, saying “speed is important, but when analyzing proteins, the biggest challenge is getting quality data.”
The growing impact of proteins as efficacious drugs and diagnostic biomarkers is forcing the analytical community to deal with extremely high levels of analyte and sample complexity. Due to the time and cost associated with developing an ELISA, mass spectrometric approaches are playing an increasing role in protein analyses. Furthermore, mass spectrometric methods enable detection of isoform variations and post-translational modifications; identification of these features being key to an understanding of protein function and activity.
Proteins exist in complex cellular environments. Reducing sample complexity prior to mass spectrometric analysis is a necessary evil. Necessary, in that if left undone you run the risk of exceeding the analytical limitations of the instrument and getting an answer you can’t trust. Evil, in that reducing sample complexity can be complicated, always involves multiple steps, and is often a highly variable process. This leaves us with answer quality being dependent on the simplicity of the sample being analyzed and the variability of the sample-preparation process. In short, we need simple solutions that give us simple solutions.
Automating Workflows
Many protein quantitation and biomarker validation procedures utilize an immunoaffinity enrichment step to purify the sample and maximize the sensitivity of the corresponding liquid chromatography tandem mass spectrometry measurements. In order to generate surrogate peptides with better mass spectrometric properties, protein enrichment is followed by a proteolytic cleavage step. Unlike ELISA, this is a serial process and a three-day sample-preparation procedure is often part of performing a single analysis.
The Perfinity Workstation automates protein sample preparation reducing process times and more importantly, producing quality results. A key to quality is having extraction, buffer exchange, digestion, desalting, and reverse-phase separation functionalities built into and performed using a single instrument.
When multiple pieces of equipment are used for this process, multiple pipettes are being used to transfer picogram quantities of analytes. There is difficulty removing the entire sample from microvessels, such as fraction collector tubes, and there is the possibility of contamination from the open nature of the apparatuses. In addition to the historical need for multiple apparatuses, complexities of the immunoaffinity LC/MS/MS sample preparation process have hindered broad application.
Consider the trypsin digestion step. Over the course of a trypsin digestion performed using traditional methods, you have peptides of varying solubility being generated that are allowed to interact with the walls of the reaction vessel; an increasingly large number of peptide substrates being formed that need to react with the enzyme a second or third time in order for the reaction to reach completion; substrates that are losing their 3-D structure, reducing the favorability of a complete reaction (trypsin being an endopeptidase); and an enzyme that is losing activity over the course of reaction due to incubation at elevated temperatures.
Biomarker validation should not require an understanding of peptide solubility, reaction kinetics, and trypsin activity. Key to the value of the Perfinity Workstation (patent pending) format accounts for these factors through an integration of columns, buffers, and software enabling users to shift their focus from workflows to answers.
Kicking thte Tires
A key quality metric for any automated workflow is the variability of the process. We assessed the performance of the Perfinity Workstation by measuring the variation occurring at each step of the process.
Affinity Capture/Automated Pull-downs.The variability of affinity selection is reduced by enhancing binding kinetics and optimizing recovery (Figure 1).
With the Workstation, affinity selection can be performed in the sample vial such that immune complex formation occurs in solution or in the column (forcing the target through a tunnel of immobilized antibody). The advantage of solution-based incubation is that this system functions in the same way as your immune system, uninhibited by immobilization.
While almost any affinity selector can be used, the use of biotinylated polyclonal antibodies is preferred. Polyclonals typically have higher binding constants, and while prone to cross-reactivity, the loss in selectivity is compensated for by leveraging the resolution of the reverse-phase chromatography performed prior to analysis. What may be the largest advantage of polyclonal antibodies is that they enable you to pull down whole classes of proteins such that all isoforms of a given protein are being captured.
Enrichment. The purification process involves the removal of hundreds of thousands of protein components. Non-specific binding in this type of format is always going to be a concern. However, adsorption is a very weak binding interaction (Kbs of ~104M), while antibodies bind very strongly to antigens (Kbs of ~109M). High degrees of rapid washing enable users to remove proteins that nonspecifically bind (adsorb to) to the antibody.
Recovery. When antibodies bind, it is the result of immunologically tuned interactions that utilize the varying properties of the 20 amino acids (hydrophobicity and charge oriented in an optimized 3-D structure) to generate binding pockets. Recovery is based on the ability to rapidly eliminate the cooperative binding properties of these interactions. Perfinity Optimized Buffers account for the wide variety of interacts that influence antibody binding, allowing for high levels of recovery and reproducibility.
Automated Trypsin Digestion. A dizzying number of interactions affect traditional solution based trypsin digestion. Many of the problems and limitations of solution-based digestion can be eliminated by rapidly pushing the reaction to completion.
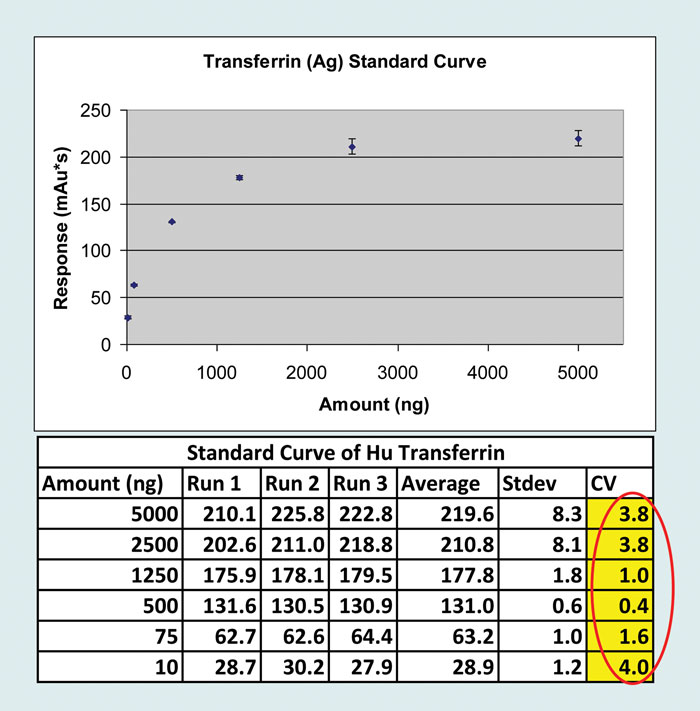
Figure 1. Affinity capture/automated pull-downs: The variability of affinity selection is reduced by enhancing binding kinetics and optimizing recovery.
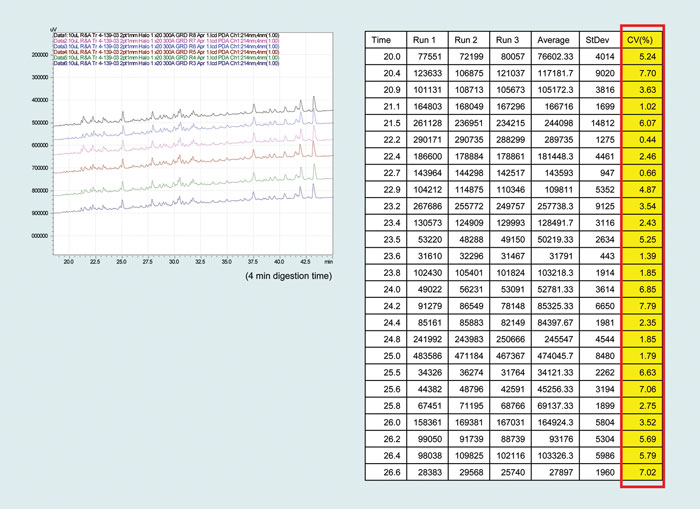
Trypsin digestion is most widely achieved by incubating the protein mixture with a 50:1 mass ratio of protein:trypsin for a 24-hour period (Figure 2). When more trypsin is used per mass of protein, trypsin begins to autodigest contaminating the sample fragments and reducing enzyme activity.
The Perfinity Trypsin Column contains immobilized trypsin. Immobilization prevents autodigestion making it is possible to use a large excess of trypsin enabling rapid, complete digestions. Hydrophobic domains buried in the structure of the whole protein are exposed during digestion resulting in peptides of low solubility. Recovering these peptides is optimized to enhance solubility without impacting reverse-phase retention. Early on in the development of our system, carryover greatly affected the reproducibility of our results, but further optimization of the Perfinity Digest Buffer eliminated this problem.
Buffer exchange and desalting. The immunoaffinity LC/MS/MS process also includes a buffer-exchange step where eluent buffer is exchanged for digest buffer. Lyophilization, dialysis, and spin column techniques are all prone to sample loss due to the surface interactions and the open nature of these systems. Also, when proteins are eluted from affinity-based capture columns, they are denatured exposing hydrophobic domains in a similar manner as described previously.
Many research groups have struggled with recovering denatured proteins that have had the opportunity to interact with surfaces. Keeping this in mind, Perfinity created a flow-through column containing a hydrophilic skin that repels proteins, and the Perfinity Digest Buffer was optimized to reduce the impact of electrostatic forces between charged amino acids ensuring quantitative transfer from between steps.
The final step prior to LC/MS/MS analysis is the desalting of dilute peptides. Given the dilute nature of the peptides being eluted from the enzyme reactor it is important that the column material be hydrophobic enough to retain diluted peptides without being overly hydrophobic resulting in retention that would exceed that of the analytical column and produce band spreading. These considerations came together resulting in a system with low variability (CVs below 10% for a five-step process.
Protein analyses involve high levels of analyte and sample complexity. Quality results are dependent on an understanding of binding kinetics, recovery, solubility, activity, retention, and a variety of other factors. Key to the value of the Perfinity Workstation format is accounting of these factors through an integration of columns, buffers, and software enabling users to shift their focus from workflows to answers.
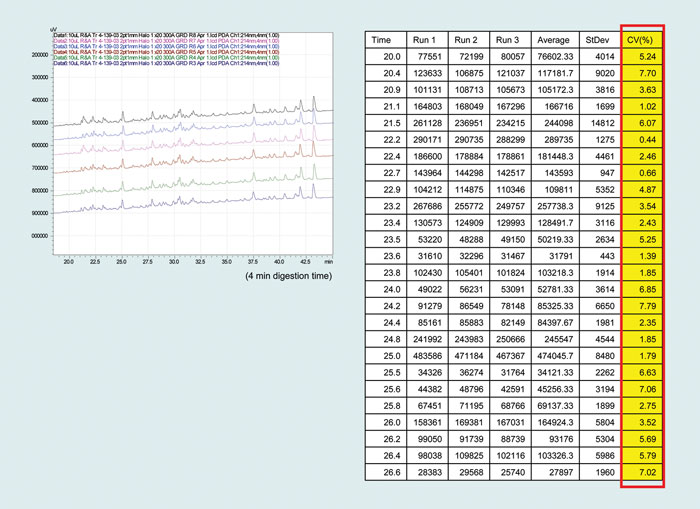
Figure 2. Variability of digestion: Transferrin digested six times and evaluated by reverse-phase chromatography. These results correspond to the first 25 peptides of the first three runs.
Kevin Meyer ([email protected]) and Nick Herold are research scientists at Perfinity Biosciences.