March 15, 2009 (Vol. 29, No. 6)
Multistep Approach Required to Thwart Ubiquitous Contaminants
Mycoplasmas, Acholeplasmas, and Ureaplasmas all belong to the Mollicutes class—small prokaryotes that do not have a rigid cell wall. Contamination of cell culture media by these organisms has become a recurring problem for the biopharmaceutical industry. In the 80s, Acholeplasma laidlawii was found to be a major contaminant of animal-derived serum. This resulted in the only somewhat successful use of 0.1 µm rated membrane filters to separate the potential mollicute contaminants.
Spongiform contamination dominated the 90s, resulting in a switch from animal-originated media to plant peptone-derived cell culture media. This change, however, caused the reappearance of mollicute contamination. This time the contamination was not restricted to Acholeplasma laidlawii, it included Mycoplasma orale, pneumoniae, fermentans, arginini, salivarum, hominis, and hyorhinis. As a result, the incidence of mollicute contamination increased significantly and created heretofore unseen problems for the biotechnology industry.
Depending on the information source, the contamination rate of secondary cell cultures varies between 15 and 30% in the U.S.; for primary cell cultures, the contamination rate is 1%.
Preventive Measures
Detection of a potential contaminant is the first line of defense. The detection of a likely contaminant makes it possible to define specific actions against it and thwart it in the future. Unfortunately, the direct assay detection of mycoplasmas, as per FDA’s Points to Consider Document (1993), is slow. The guidance document describes both agar and medium inoculation.
No less than 0.2 mL of product sample is spread over at least two agar plates and is incubated for at least 14 days at 36±1ºC at an anaerobic atmosphere. A >10 mL sample is introduced into 50 mL of medium (Hayflick) and incubated. Samples of the medium are taken at day 3, 7, and 14 and sampled on agar plates and incubated—the minimum time to determine a contamination is 28 days. This drawn-out detection process causes delayed cell culture media releases, which ultimately, impacts product releases as well.
Corresponding to the direct agar/broth method, an indicator cell line test is also recommended. Vero or 3T6 cells are employed, which are inoculated by a >1 mL sample and incubated for 3–5 days at 36±1ºC in a 5% carbon dioxide atmosphere. The cells are fixated and stained utilizing Hoechst stain, which is DNA binding. The identification of contaminating mollicutes is done by fluorescent microscopy.
PCR represents the fastest and, probably, the most reliable rapid detection method available. Depending on the PCR method, sensitivity and speed vary; Touchdown PCR provides results within 5–8 hours with a sensitivity of 1 cfu/mL. A major disadvantage of any PCR method is the fact that it cannot distinguish between living or dead cells, which can result in the rejection of usable media. On the other hand, the speed, accuracy, and broad detectability represents an excellent opportunity for go-no-go decisions to be made before media is supplied into the process.
Inactivation of the contaminant is the next preventive measure. Comparable to viral clearance, the best approach to process fluid safety is a multistep, orthogonal inactivation and removal practice. Typical inactivation steps for mycoplasma contaminants can be the use of antibiotics (Gentamycin is effective to a certain degree), heat treatment at 45ºC for 30 min or 60ºC for 10 min, radiation, or pH shift.
Heat, pH, and radiation can alter media components, though. Heat is mainly introduced by the use of 80ºC water for injection, into which the powdered media is mixed and solubilized. Contact time and uniformity of the mixing process are essential for the heat inactivation step. Mycoplasmal contamination has been reported after heat treatment, however, this could be due to insufficient mixing. The use of radiation has been found to produce a reduction of viable mycoplasma > log 8 at a radiation dose of 25–35 kGy. Nevertheless, any of these steps require validation to verify the inactivation robustness versus the media product stability and efficacy.
Removal
Mollicutes removal is most commonly accomplished by filtration with 0.1 µm rated membrane filters. This filtration step requires process validation to assure the 0.1 micron rated filter performs as desired. The influence of process parameters—viscosity, differential pressure, contact time, fluid volume filtered, and organism species —determines the retentivity of the filter.
The variability of these factors makes it necessary to perform process validation, including product and process parameter challenge tests. Studies have shown that 0.1 um rated membrane filters that were able to retain challenges of >log 7 per cm2 of Acholeplasma laidlawii were unable to reach the same level of retentivity with Mycoplasma orale or M. pneumoniae.
The retention capabilities of different filters varies greatly, in fact, some filter vendors only guarantee proper reduction levels at specific higher Bubble Point values. Adsorptive retention is effective with some membrane polymers (Figure), but can cause unnecessary fouling, i.e., premature blockage. Additionally, adsorptive retentivity is affected by a multitude of process parameters and is, therefore, fairly unreliable.
Process validation also has a specific importance with 0.1 µm rated filters, as there is no standardization of the retentivity of such filters, which means variability in the performance of different 0.1 µm rated filters. A Parenteral Drug Association Mycoplasma Task Force is working on a standard challenge test proposal that could provide recommendations for the end-user’s testing of available 0.1 um filters.
Such tests could be performed in a laboratory setting, but retention reliability has to be performed with the actual product under process conditions. This approach, already being utilized with 0.2 um rated and viral removal filters, will provide assurance that the chosen filter is an appropriate one for the specific application. Redundancy or pure reliance on filter manufacturer’s data will not suffice.
The change from animal-derived media to plant peptone-derived media caused a recurrence of mycoplasmal contamination in cell culture media. This resurgence has resulted in a problem for the biotech industry, which relies heavily on large volumes of cell culture media for mammalian culture processes. A single preventive action will probably not prove adequate to assure contaminant-free medium; a multistep approach is necessary. Such an approach must be embraced by the end-user, and the raw material supplier must share the burden and enhance assurance.
Current detection, inactivation, and removal methods, in conjunction with appropriate process validation are the best options to prevent mycoplasma contamination.
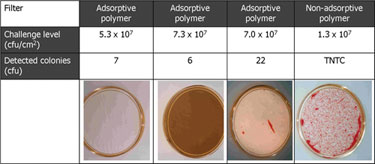
Effect of adsorptive retention and pore structure
Maik W. Jornitz ([email protected]) is vp product management at Sartorius Stedim Biotech. Web: www.sartorius-stedim.com. Theodore H. Meltzer, Ph.D., is from Capitola Consultancy.



