November 15, 2008 (Vol. 28, No. 20)
HTRF Technology Finds Applications in GPCR, Kinase, and Sirtuin Detection
Homogenous time-resolved fluorescence (HTRF) entered the drug-screening world about ten years ago. Now, researchers use it to quickly and sensitively detect GPCRs, kinases, new biomarkers, protein-protein interactions, and other targets of interest. Such applications were the topic of presentations at the “HTRF in Drug Discovery” symposium in Avignon, France, sponsored by Cisbio Bioassays. The goal of the meeting was “to give a snapshot of the most up-to-date findings and applications of HTRF in drug discovery,” said Francois Degorce, conference chair and head of marketing at Cisbio Bioassays, which was recently acquired by IBA.
HTRF is a highly sensitive and stable platform for the detection of molecular interactions, according to Degorce. “HTRF fluorophores expedite assay development, and they are suitable for miniaturization, scale-up, and high-throughput screening under cell-based conditions, giving more relevant results.”
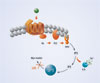
For instance, “in the world of GPCRs, we have mastered the configuration of IP1,” explained Degorce, referring to a downstream metabolite of IP3 (inositol triphosphate) that accumulates in cells following stimulation of GPCRs (Figure 1). Cisbio’s IP-One HTRF® Assay is designed to measure IP1 in HTS applications. “Kinase applications remain good friends of HTRF,” Degorce noted, and Cisbio’s line of HTRF KinEASE™ kits measure threonine, serine, and tyrosine kinase activity (Figure 2).
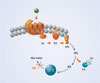
Figure 1. Gq signaling pathway

Figure 2. HTRF KinEASE assay protocol
Second-Generation Terbium Products
Cisbio’s first-generation products use Europium cryptate to monitor reactions between biomolecules. In April, Cisbio launched its HTRF Terbium (Tb) platform for GPCR screening with the introduction of IP-One Tb and cAMP Tb. These assays are the first to incorporate Lumi4™-Tb cryptate for enhanced screening performance. Lumiphore developed Lumi4-Tb and licensed it exclusively to Cisbio for second-generation drug discovery assays.
Terbium cryptate shows different photophysical properties than Europium, and it is compatible with additional acceptors, including green-emitting ones. This allows for multiplexing of signals. Moreover, all Terbium products run on the same HTRF-compatible instruments as Europium ones.
Terbium is exceptionally bright—10 to 20 times brighter than Europium—lending itself to the exploration of cell surface receptors, according to Eric Trinquet, head of Cisbio’s technology and chemistry department. In proof-of-concept experiments, done in collaboration with researchers at the Institute of Functional Genomics, Trinquet found that ligand-binding assays that are selective for cell-surface receptors run more efficiently with Terbium than Europium cryptates. Additionally, Terbium assays run in much more cell-friendly experimental conditions than Europium assays, he noted.
“These new cryptates also perform outstandingly when used with suicide enzyme-labeling technologies like SNAP Tags from Covalys,” Trinquet said. His group has developed new substrates with enhanced reactivity for GPCR ligand-binding and oligomerization assays in living cells.
Cisbio is developing a new HTplex™ platform for the detection of two biological agents in the same microwell. The power of Terbium to multiplex was demonstrated in an experiment conducted in collaboration with Novartis, which aimed to identify the normal and mutant forms of the Huntington’s disease protein in neuronal cell lysates.
By using a common Terbium-labeled antibody directed against a conserved epitope on the protein and two other antibodies labeled with red and green acceptors, both the normal and mutant form of the Huntington’s protein were detected simultaneously (Figure 3). In this system, the Terbium cryptate acts as a fluorescence donor for both acceptors, and no overlap of their fluorescent signals occurred. “This first proof-of-concept of the HTplex technology worked well,” said Trinquet.
Cisbio will launch HTplex services by the end of this year, and new tools for exploring cell surface receptors should be out in 2009.

Figure 3. HTplex Hungtintin protein assay scheme
Drug Discovery and Quality Control
Other researchers described their experiences with Cisbio’s HTRF products as alternatives to existing drug discovery methods. Martin Rowlands, Ph.D., research support for the analytical technology and screening team at the Institute for Cancer Research (ICR), uses the HTRF KinEASE™ kit to profile and screen serine/threonine kinases involved in cancer-cell signaling. Inhibitors of such kinases have value as cancer drugs. Dr. Rowlands and his colleagues validated a particular mitotic kinase as a drug target, which is found in the early stages of breast cancer and contributes to relapses. There is no protein substrate, however, available for this threonine/serine kinase or specific antibodies.
Dr. Rowlands tried the HTRF-KinEASE SKT kit that provides three peptide substrates validated for more than 100 kinases. One substrate showed good concentration-dependent activity in the initial screening. The team automated the process to screen 80,000 compounds in the ICR’s library and produced 60 hits. Cisbio’s HTRF Transcreener® ADP assay also has helped Rowlands to detect other kinases and heat shock proteins that are of interest in cancer progression.
“Overall, HTRF kits provide a reliable and robust assay method for both primary screening and hit-to-lead applications,” Dr. Rowlands said. Cisbio’s HTRF kits can be applied to a number of kinases and heat shock proteins, including those with unknown substrates.
Thomas McDonagh, research scientist at Elixir Pharmaceuticals, described the adaptation of HTRF to study sirtuins, a family of histone deacetylases that regulate gene transcription. Elixir focuses on finding inhibitors of human SIRT enzymes, such as SIRT1, that play a role in cancer, HIV, metabolic disease, and aging.
“What’s been lacking in this field is a true understanding of the nature of these enzymes,” said McDonagh. Small molecule modulators found through HTS could be used to understand the underlying biology of sirtuins. Elixir scientists have used the Fluor-de-lys assay, which although HTS, suffers from the disadvantage of being highly sensitive to fluorescent artifacts. They also developed an in-house nicotinamide release assay, but its throughput is slow.
Alternative HTRF Assays
As an alternative, Sara Bdioui, short-term project manager at Cisbio, helped Elixir researchers design an HTRF assay that is suitable for screening SIRT1 and other sirtuins. The assay takes 30 minutes for completion, operates efficiently at picomolar concentrations, and is very flexible at using different substrates, she said. Elixir will use the assay for general routine investigations and to screen libraries to characterize hit-to-lead compounds.
One of the assay’s greatest advantages is its cost. McDonagh calculated that the HTRF assay costs $0.83 per assay point, compared to $1.94 for the Fluor-de-lys assay, and $3.50 for the nicotinamide assay. “HTRF is a much cheaper assay in the long term,” said McDonagh.
Pharmaceutical companies now focus on about 500 known drug targets, yet the human genome’s 30,000 genes translate into 500,000 proteins that could be potential drug targets. “There should be a lot more targets available for drug discovery,” said Wei Zheng, Ph.D., group leader at the NIH Chemical Genomics Center.
Dr. Zheng’s group adapted Cisbio’s IP-One HTRF Assay to measure the activity of five Gq-coupled GPCRs in 1,536 well microplates for primary HTS. They compared IP-One to a traditional intracellular calcium release assay that requires fluorescent dyes and expensive kinetic plate readers. Dr. Zheng described the steps taken to optimize the IP-One assay, such as using adherent cells rather than suspended cells and eliminating a wash step. The activity of antagonists determined in the IP-One assay correlated well with measurements in the intracellular calcium assay, but agonist activity varied for cell lines.
“IP-One offers a viable alternative method for primary screening of Gq-coupled GPCRs,” Dr. Zheng concluded. Whereas traditional screening takes four to six months, HTRF reduced the process to one to two months.
Increased Productivity
In addition to aiding researchers who investigate new drug targets, HTRF lends itself to monitoring the manufacturing of biotherapeutics to insure safety and quality. At OncoMed Pharmaceuticals, Esohe Idusogie, Ph.D., head of the analytical group in process development, replaced traditional ELISA assays with HTRF technology to identify the top antibody-producing clones for monoclonal antibody production. “That had an enormous impact on our productivity,” she reported. In fact, the switch to HTRF increased productivity by several hundred orders of magnitude. Now Dr. Idusogie is looking for ways to extend HTRF to process analytical technology (PAT).
Traditional PAT tools such as ELISA, quantitative PCR, electrophoresis, and 2-D gels are slow and cumbersome. “They are not PAT friendly,” Dr. Idusogie explained. She is applying HTRF to monitor host cell proteins, host cell DNA, and leached protein A. These three main types of impurities that arise during the production of monoclonal antibodies are of regulatory concern because they contribute to immunotoxicity. Direct HTRF binding assays to detect them appear to be feasible, Dr. Idusogie added.