October 15, 2009 (Vol. 29, No. 18)
Technology Acquired from Elan Enables Company to Convert Intravenous Drugs to Oral Formulations
The drugs in the pipeline at Merrion Pharmaceuticals are not new chemical entities poised to become blockbuster drugs. Instead, the company specializes in designing oral formulations for existing drugs that must be given intravenously or by injection. Not only do patients prefer to swallow a tablet, but oral drugs can be safer and less expensive than those requiring a needle for delivery. “We see huge opportunities in taking various efficacious intravenous drugs and converting them to an oral formulation,” says John Lynch, CEO.
At a recent annual meeting of the American Society of Clinical Oncology, Merrion announced positive results from a Phase II trial of Orazol™, a tablet form of zoledronate, currently available only as an intravenous (IV) infusion.
Zoledronate, a bisphosphate, is primarily used to protect against bone fractures in people with advanced cancer that has metastasized to the bone. The study involved prostate cancer patients with proven bone metastases treated at 13 sites in the U.S. and Europe. Levels of bone biomarkers showed that weekly therapy with Orazol tablets was as therapeutically effective as monthly IV infusions of zoledronate.
“We are very pleased with these results. We saw the therapeutic effect after the first tablet, and side effects were minimal,” says Lynch. The company is looking for a strategic partner to move Orazol into Phase III trials and market the product.
More than three million people worldwide have been treated with IV infusions of zoledronate. Patients with prostate, breast, lung, and renal cancer and multiple myeloma are especially susceptible to bone fractures. Zoledronate also is prescribed for osteoporosis and Paget’s disease, and sales of the drug reached $1.65 billion in 2008.
Orazol tablets offer several advantages over IV infusions, Lynch explains, including fewer toxic side effects and injection site complications, more flexible dosing options, reduced medical costs, and increased patient convenience such as taking medications at home.
Moreover, recent studies by other researchers show that zoledronate improves survival of some cancer patients. In a recent study published in the New England Journal of Medicine, zoledronate added to endocrine therapy in women with early breast cancer increased disease-free survival by 33%, compared to women on endocrine therapy alone. Generally, only some late-stage cancer patients receive zoledronate as a supportive treatment for bone metastases. If more studies confirm the anticancer effects of zoledronate in early-stage cancer, “an oral tablet would be an ideal way to treat more types of cancer patients,” Lynch says.
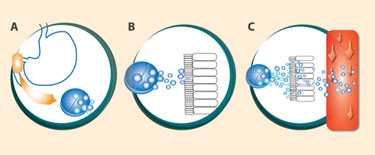
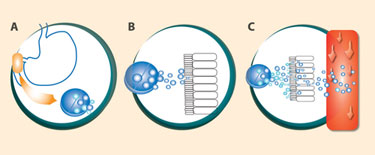
GIPET reportedly improves the delivery of poorly permeable compounds to the bloodstream after oral administration.
Acquisition of Technologies
Lynch, Michael McKenna, and Thomas Leonard, Ph.D., cofounded Merrion in 2003 to advance platforms that they acquired from Elan when it moved into biopharmaceuticals and divested its drug-delivery technologies.
Among the acquisitions was Merrion’s GIPET® technology, which makes it possible to convert formerly injectable drugs into tablets. GIPET, short for gastrointestinal permeation enhancement technology, uses food additives listed on the FDA’s GRAS (generally recognized as safe) list to enhance drug absorption. Merrion patented a range of ingredients that are combined with a drug and then coated into a tablet, which is then delivered to the duodenum. As the tablet dissolves, the drug and absorption enhancer are released and absorbed. Studies show that GIPET significantly improves the bioavailability of oral drugs—up to 46 times for some drugs, Lynch says.
Because GIPET is based on GRAS ingredients, it may be easier to obtain FDA approval for Orazol and related drugs, Lynch believes. Under the FDA’s 505(b)2 regulation, the FDA could base its approval for the oral formulation partly on findings used to gain approval for the original intravenous drug. This process would greatly reduce the time to market and expense of developing Orazol and other drugs in Merrion’s pipeline.
GIRES™, another technology acquired from Elan, consists of a controlled-release drug dose stored in an inflatable pouch, which is wrapped inside a capsule. The pouch is thinner and more pliable than a soft contact lens. When stomach acid chews through the capsule, a gas-generating system inflates the pouch and slowly releases the drug for 16 to 24 hours. “To the best of our knowledge, this is the longest retention time reported in the stomach,” Lynch says. Company researchers are testing some medications in GIRES, but the company’s immediate focus is to advance the GIPET technology.
Oral Diabetes Drugs
In November 2008, Merrion teamed up with Novo Nordisk to develop and commercialize oral formulations of Novo Nordisk’s insulin analogues using the GIPET technology. “Oral insulin is considered the holy grail of drug-delivery technology,” says Lynch, who adds that Novo Nordisk was so pleased with the results achieved to date, that it is pursuing a tablet-based diabetes treatment on a large scale.
“Many people are skeptical about oral insulin, because people have worked on this for many years and no one has succeeded,” Lynch explains. He believes that an oral insulin formulation is within reach, based on the combination of Merrion’s technology that allows oral peptide drugs to be absorbed efficiently and Novo Nordisk’s expertise in engineering insulin. Additionally, both companies agreed to work together earlier this year to develop and market oral formulations of Novo Nordisk’s GLP-1 agonists.
Other drugs in Merrion’s pipeline include Almerol, an oral bisphosphonate for the treatment of osteoporosis. Almerol has completed Phase II trials. The GIPET technology, Lynch says, provides similar absorption, but at just 8% of the dose, compared to current formulations, thereby improving side effects and simplifying dosing.
Acyline, an oral drug for prostate cancer, is in Phase I trials. If successful, acyline will be the first oral product in the area of gonadotropin-releasing hormone analogs, Lynch reports. In preclinical testing is MER 102, an oral anticoagulant that could replace daily injections. “We’re encouraged by what we have accomplished to date. As we move forward, our technology is proving to be applicable to many areas,” says Lynch.