October 1, 2012 (Vol. 32, No. 17)
Novel Approach Produces Gram-Per-Liter Expression Levels of Biologically Active Proteins
Recent advances have allowed transient protein expression to become a fast, flexible, and economical way to produce high-quality recombinant proteins without the time and cost associated with generating stably transfected cell lines.
The levels of protein generated via transient expression, however, have tended to be significantly lower than those of stably transfected cell lines. When large amounts of protein are required, stable cell lines are often still preferred to transient systems.
To further increase the utility of transient expression systems, the next key advances will need to approach, or equal, expression levels attained using stable expression systems without losing the speed and flexibility of transient systems.
In this article, we report the development of a novel transient transfection system—Expi293™—that utilizes high-density 293F cell cultures to generate expression levels greater than 1 g/L for human IgG and non-IgG proteins.
System Optimization
To obtain multifold increases in protein expression levels over traditional transient systems, it is critical that the transfection step be performed at significantly higher cell densities, allowing for increased volumetric protein production capacity. This requirement of high density cell cultures at the time of transfection necessitated the development of a new cell culture medium able to support extremely high numbers of cells, with high viability, throughout the production run.
To this end, a novel chemically defined and animal origin-free cell culture media (Expi293 Expression Medium) was developed that allows 293F cells to reach viable cell densities of 14 x106 cells/mL or greater while still maintaining high viability (Figure 1A).
In contrast, traditional 293 culture media support a maximum viable cell density of only 4–5 x 106 cells/mL. This new cell culture media enables transfection at 2.5 x 106 cells/mL, compared with traditional protocols that recommend transfection at 1.0 x 106 cells/mL.
This increase in the number of viable cells/mL at the time of transfection enhances the protein production capacity per mL of culture media by 2.5-fold and leads to significantly higher volumetric protein yields.
In addition to allowing for increased viable cell densities in culture, cell-specific productivity was also improved through the generation of a high expressing HEK293F cell line and by the development of transfection enhancers that are added directly to the media post-transfection.
To generate the high-producing Expi293F cells, parental FreeStyle 293F cells were adapted into Expi293 expression medium and then selected over time for superior protein production under high-density culture conditions.
The resultant Expi293F cells possess an increased growth rate compared to FreeStyle 293F Cells (average doubling times of 21–24 hours vs. 25–27 hours, respectively), higher viable cell densities, and increased cell viability.
The new Expi293F cells produced up to 1.7-times more protein than parental FreeStyle 293F cells used in the Expi293 expression system (Figure 1B), indicating a significant increase in the specific productivity of the Expi293F cells.
Lastly, the transfection reaction itself was optimized through the development of a new transfection reagent (ExpiFectamine™ 293) paired with proprietary transfection enhancers designed to work specifically with this reagent to further increase overall protein yield by approximately 3-fold (Figure 1C).
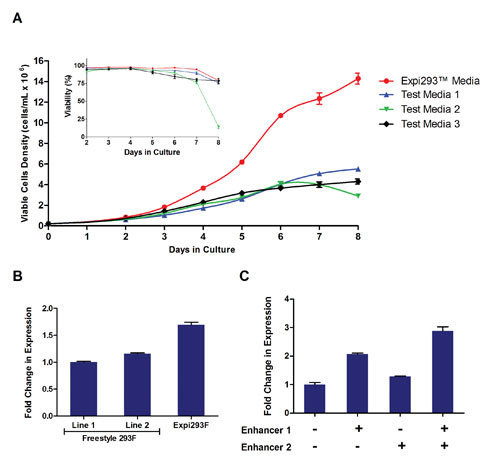
Figure 1. Optimization of cell culture medium, cell line, and transfection enhancers: (A) Expi293F Cells were seeded at 0.2 x 106 cells/mL in various culture media and cell density and viability were monitored over time. (B) Human IgG was expressed using the Expi293 Expression System using three different 293F cell lines, (C) with and without the addition of transfection enhancers 1 and 2.
Gram per Liter Expression
When combined into a single expression system and optimized via multi-factorial DoE, the protein yield improvements enabled by each of the Expi293 system components (media, cells, transfection reagent, enhancers), had an additive effect leading to significant increases in protein expression levels compared to the FreeStyle 293 expression system.
Expression levels of greater than 1g/L were attained for multiple proteins, including a human IgG and an Fc-tagged Cripto protein (Figures 2A–B). The largest protein yield increase, 11.8-fold, was seen in the expression of a rabbit monoclonal antibody (Figure 2C). A 3.5-fold increase was observed for the integral membrane protein b-2-adrenergic receptor (Figure 2D), and a 4.4-fold increase for the secreted growth factor erythropoietin (data not shown).
These results were readily scalable, with consistent protein expression levels generated in formats ranging from 1 mL cultures in 24-well plates to 1 L cultures in shaker flasks with successful protein expression also obtained in 96-well plate formats.
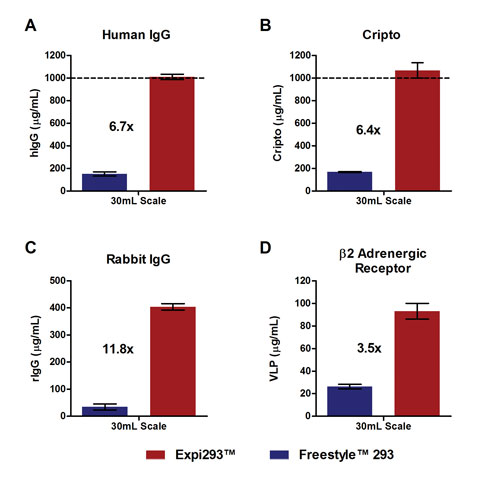
Figure 2. Comparison of expression levels for four different proteins using the Expi293 system and the FreeStyle 293 System
Protein Functionality and Glycosylation Pattern Assessment
Although ultra-high protein yields are desirable, the resultant protein is less valuable if it is aggregated, misfolded, degraded, or improperly glycosylated, thus requiring significant additional purification efforts to yield a final protein product with high purity. The quality of the proteins expressed in the high-density Expi293 system were compared to the quality of the same proteins expressed in the FreeStyle 293 expression system in terms of biological activity, binding, and glycosylation patterns.
Recombinant erythropoietin (EPO) was tested for biological activity using a TF-1 cell-based proliferation assay. The biological activity of EPO was highly similar for both expression systems, with an EC50 value of 0.45 ng/mL for EPO made in the Expi293 system vs. 0.58 ng/mL for the FreeStyle 293 system (Figure 3A).
The ligand-binding activity of β-2 adrenergic receptor was equivalent for both expression systems, with Kd values of 1.069 vs. 1.057 nM and Bmax values of 30.1 vs. 29.1 pmol ligand/mg for β-2 adrenergic receptor produced in the Expi293 and FreeStyle 293 systems, respectively.
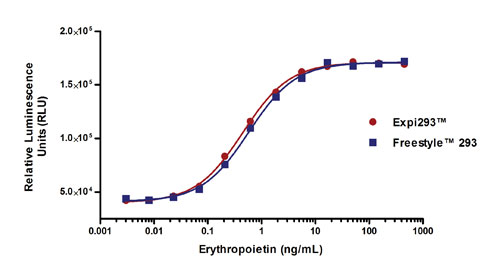
Figure 3A. Assessment of biological activity and glycosylation profiles of proteins generated using the Expi293 System and the FreeStyle 293 System. Human erythropoietin was expressed using the Expi293 and FreeStyle 293 Expression Systems and tested for biological activity using a TF-1 cell-based proliferation assay.
Additionally, the binding of an anti-GFP antibody for its target protein in an immunoblot was demonstrated to be equivalent for antibody produced in both expression systems (data not shown).
Analysis of the N-linked carbohydrates for a recombinant human IgG produced in both the Expi293 and FreeStyle 293 systems showed that the glycosylation profiles were highly comparable for antibody made in either system (Figure 3B).
For both proteins, the G0F structure was the most prevalent, followed by G1F, with these two structures accounting for approximately 85% of the total N-glycans in each case.
Antibody fucosylation levels have the potential to impact antibody Fc-related effector functions; no differences in the levels of GO (nonfucosylated) variants were observed between the systems. These data indicate that the quality of the protein expressed in the Expi293 expression system, as determined via biological activity, binding, and glycosylation analysis, is equivalent to the quality of protein expressed in the FreeStyle 293 expression system.
Together, the results demonstrate that significant improvements in functional protein yields can be attained using a novel transient mammalian expression system that incorporates multiple advances in protein expression technology into a single, easy-to-use format.
High transfection efficiencies at increased cell densities, along with the incorporation of transfection enhancers, were vital in achieving multifold increases in recombinant protein expression levels.
Using the Expi293 system, protein expression levels that rival those achieved in some stably transfected cell lines were obtained in a simple and rapid transient expression system.
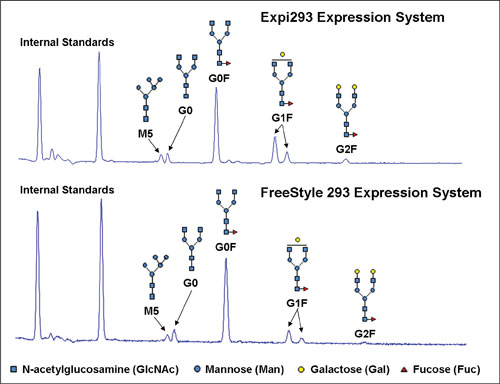
Figure 3B. Highly similar N-linked glycosylation profiles were obtained for human IgG expressed in the Expi293 and FreeStyle 293 Expression Systems.
*Meredith B. Jones, Ph.D., *Chao Yan Liu, *Sanjay Vasu, Ph.D., and *Isabel Cisneros are scientists, Henry Chiou, Ph.D. ([email protected]), is a senior product manager, and Jonathan F. Zmuda, Ph.D., is an associate director, R&D at Life Technologies. The authors would like to thank Yolanda Tennico for her expert assistance with glycosylation profiling. The Expi293 Expression System and FreeStyle 293 Expression System are for Research Use Only. Not for use in diagnostic procedures.
*These authors contributed equally to this work.


