June 15, 2007 (Vol. 27, No. 12)
Automated Technology Improves Quality and Quantity of DNA Samples
Many if not most aspects of life science research revolve around the use of molecular material from various cellular paradigms, be they animal, plant, bacterial, or fungal, for example. Therefore, the majority of research procedures are immediately concerned with and sometimes plagued by the quality of the molecular starting material. Furthermore, most of the procedures involved are still largely manual and consist of multiple steps, each one increasing the variability both within and between samples and decreasing the actual quantity of material recovered.
The most commonly used starting material is DNA for which a plethora of extractions have evolved. Most of these fall into two categories—precipitation and solid-phase capture. Both systems have their advantages and drawbacks and possess similar quality and quantity issues. To remove the human error element, automation can be introduced but is limited. Precipitation steps are difficult to automate due to centrifugation and pour out steps, and solid-phase processes are expensive to automate because of the different liquid-handling, plate-handling, and vacuum requirements.
Magnetic Particle Processor
The Cincinnati Children’s Hospital Research Foundation (CCHRF) has been using the Thermo Scientific (www.thermo.com) KingFisher 96 magnetic particle processor to significantly improve DNA sample quality and quantity as well as consistency and throughput.
Facility
The CCHRF Genetic Variation and Gene Discovery core facility, headed by Gregory Grabowski, M.D., and Mehdi Keddache, carries out a wide range of translational research protocols for gene-based studies requiring over 35,000 DNA extractions per year as well as DNA sequencing (250,000 reads/year), PCR optimizations (over 400/year), and millions of SNP genotypes generated every year.
With such a requirement for dependable, consistent, and high-speed extractions, the CCHRF implemented the Thermo Scientific KingFisher 96 instrument along with a liquid-handling robot. This has not only increased quality, quantity, and throughput but also offers the flexibility for similar separations of RNA, proteins, and cells when required.
KingFisher Technology
The Thermo Scientific KingFisher 96 uses magnetic rods to move samples bound to magnetic beads through the various purification phases—binding, mixing, washing, and incubation. This means that liquid handling during the extraction is not required since all the solutions and buffers required are prefilled into whichever arrangement is required. As a result, the KingFisher 96 can purify 96 samples in 15–30 minutes.
The magnetic rods are covered by disposable tip combs, which can be placed in two positions: over the magnet or extending from the end of the rod. With the tip comb over a magnet it can actually descend into the sample rather than to the side of the well. By doing this, it is the sample that is moved to the next buffer rather than just the solution that is changed within the same well, ensuring that there is virtually no carryover between steps.
Alternatively, with the tip comb fixed to the end of the rod, it can be used to agitate the sample without any magnetic attraction of the sample, to provide homogenous distribution of the sample in the buffer. Furthermore, sample cross contamination and reagent cross over is effectively eliminated due to the technology.
CCHRF has performed a number of evaluation processes and ongoing quality-control studies on the introduction of the Thermo Scientific KingFisher 96 using typical sources including rodent tissues, blood, buccal swabs/brushes, as well as already purified DNA needing repurification. The versatility of the magnetic bead-based protocols available also enabled CCHRF to assess a number of RNA and protein-isolation procedures.
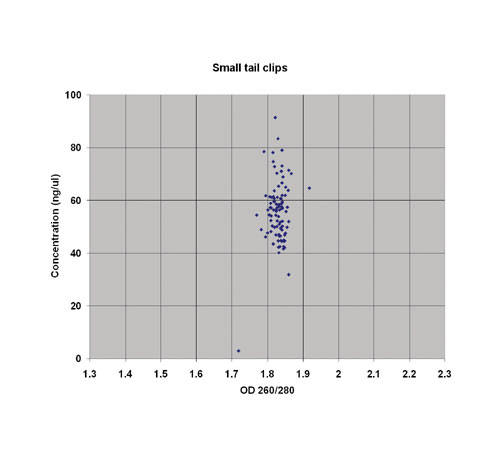
Figure 1
Rodent Tissues
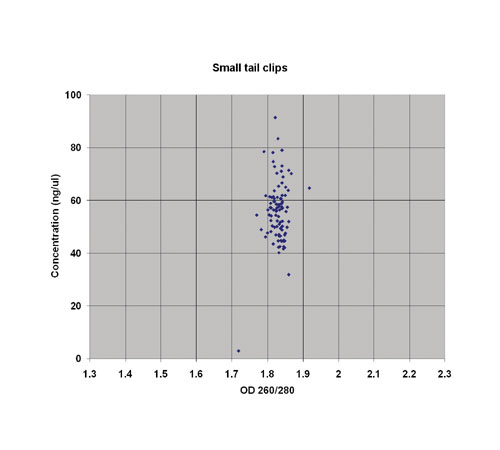
Tissue samples from tail and ear clips as well as dissected organs were lyzed in a deep 96-well plate for two hours using Proteinase K (Qiagen). Once lyzed, silica-coated magnetic beads were added, which selectively bind DNA, along with ethanol, to ensure clean separation of the DNA from the rest of the lyzate. The beads, with attached DNA, were then passed through two wash buffer steps before elution of the DNA in 200 µL of 10 mM Tris (pH 8.0).
The protocol was not only simple to implement but also robust, with no hair, bone, cartilage, or undigested tissue carry over. Furthermore the high throughput means that all 96 samples are processed in 20 minutes, and yet there is excellent day-to-day consistency and efficiency (Figure 1).
Blood Samples
Whole blood (including the buffy coat) was processed using the manufacturer’s protocol in deep 96-well plates. Using fresh blood samples, the typical recovery rates were excellent at 20–50 µg/mL blood, and even with old blood (up to six months old at 4ºC), it was typical to recover 5–15 µg/mL.
Buccal Swabs/Brushes
Buccal cell sampling procedures such as swabs and brushes generally remove few cells and therefore there is a low yield expectation (Figure 2). Despite this, these collection processes remain a popular and essentially non-invasive collection method. To process the samples, the cells were lyzed at 65°C for one hour in deep 96-well plates using a small volume of buffer. To ensure that all the sample is recovered, the swabs/brushes are centrifuged while held above the well and then removed.
The same protocol as used for the rodent tissues was run on the Thermo Scientific KingFisher 96 and produced DNA yields of 1.5–7 µg/brush. The purity of the samples produced was also high.
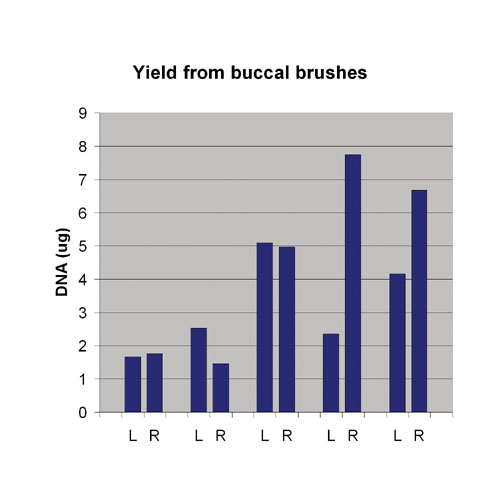
Figure 2
DNA Repurification
Genotyping assays are sensitive to the quality and purity of the DNA source. Therefore, it is sometimes necessary to repurify samples before they can be used for genotyping, but this often greatly reduces the yield. The Thermo Scientific KingFisher 96 can be used to produce 90% recovery on repurification via a simple and rapid procedure. To start with, the original DNA solution is combined with binding buffer, beads, and ethanol and then passed through two wash steps. The DNA is then eluted in 200 µL of 10 mM Tris (pH 8.0) ready for direct use in genotyping procedures (Figure 3).
This process has proved invaluable for both microsatellite genotyping assays and TaqMan (Applied Biosystems) SNP assays, providing dramatic increases in DNA performance.
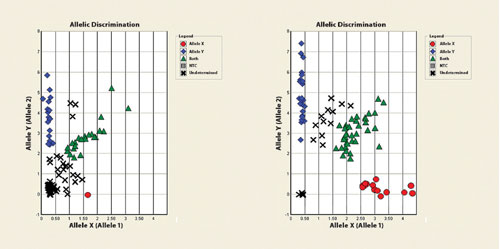
Figure 3
Comparisons
In comparison to other leading DNA purification systems used for long-term DNA storage, the KingFisher 96 performs as well if not better. It produces the same level of quality in both the raw DNA and any subsequent downstream amplifications/modifications (Figure 4).
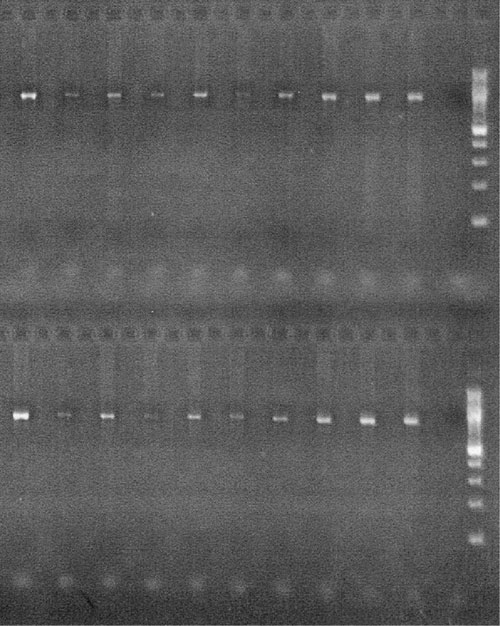
Figure 4
GoldenGate SNP Genotyping Assay
The Thermo Scientific KingFisher 96 is also being used at CCHRF for the automation of the Illumina (www.illumina.com) GoldenGate assay. The procedure allows the genotyping of up to 1,536 custom SNP markers in a multiplex fashion and uses a mobile solid-phase reaction in the form of streptavidin-coated magnetic beads binding biotin-labeled DNA. With the help of the PCR plate-sized magnets and tip combs, the procedure is not only accelerated but the risk of human error is virtually eliminated.
Conclusions
The Thermo Scientific KingFisher 96 provides a highly flexible platform for molecular and cellular purification from all possible sources. At the CCHRF, the KingFisher 96 is used for a growing number of applications, mostly focused around DNA but also including RNA and protein purification.
Validation studies and ongoing quality assessment have shown that the KingFisher 96 produces reproducible quality with increased yields and speeds in an automated environment, when compared to previously used protocols. All methods transferred to the KingFisher 96 are comparable to the present gold standards and ensure that all research at CCHRF is conducted using the highest quality starting material.
Arja Lamberg, Ph.D., is product line manager at Thermo Fisher Scientific.
Web: www.thermo.com.
E-mail: [email protected].