April 15, 2011 (Vol. 31, No. 8)
Speeding Up the Process Has Increased the Technology’s Utility in Systems Biology
Creating solutions driven by focused systems biology questions demands new technological approaches. Automation of detection systems has allowed biologists to move beyond the limits of manually driven tools. One technology that has not traditionally been easy to automate is flow cytometry. New developments have led to the emergence of flow cytometry as a remarkable tool in automated high-content screening. The process requires the optimization and standardization of many procedures and, importantly, solutions for rapid and robust analysis that match the time savings generated by automation.
Perhaps the most difficulty is encountered in the need to quickly reduce very large datasets into something meaningful. Most current procedures operate in a purely vertical fashion that does not translate well into large volume processing. Furthermore, flow cytometry is a field in which the typical software package is a complex tool designed for universal use, not to handle assays based on high well number plates, particularly those with 384 or more wells. Even with 96-well plates, most flow-cytometry analysis approaches are inefficient and cumbersome.
The approach we propose requires the creation of robust, module-specific tools focused on solving specific analytical problems. For example, if the need is for IC50 values, then tools can be designed for high-speed flow-cytometry analysis with direct output of IC50 values from raw list-mode data. Such a tool may be highly efficient for this application, but not effective for a different assay.
The fundamental approach can be modified from one application to another, but this does require alteration of the software. For HT flow to be efficient and adopted, software specific for a given application may be required. As an example, for drug screening, we have designed a robust process that uses a combination of plate-design profiles, plate-analysis profiles, and predesignated outputs. A second approach has been designed for immunophenotyping applications.
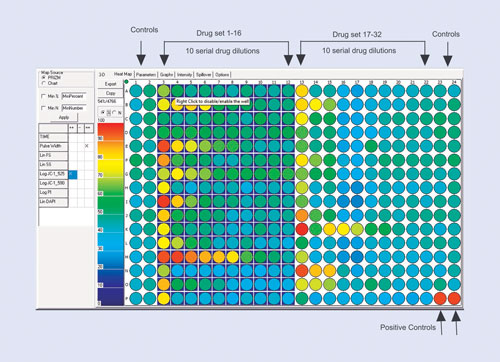
The drug-screening example is shown in Figure 1. Here the assay design is optimized for a 384-well plate and for 10-point drug dilutions. The most efficient way is to use two blocks of 16 drugs, leaving four columns available for negative and positive controls. This efficient design allows 32 drugs to be tested on a 384-well plate.
To ensure high-quality control, together with necessary speed, all processes are performed on automated systems (Biomek®, Beckman Coulter). Thus cells, reagents, dyes, and chemicals are robotically added to plates, and of course, plates are run on the flow cytometer (CyAn®, Beckman Coulter) supplied by an automated robotic delivery system (HyperCyt®, IntelliCyt). Automation at the preparative level brings good quality control, reproducible results, and economy of scale.
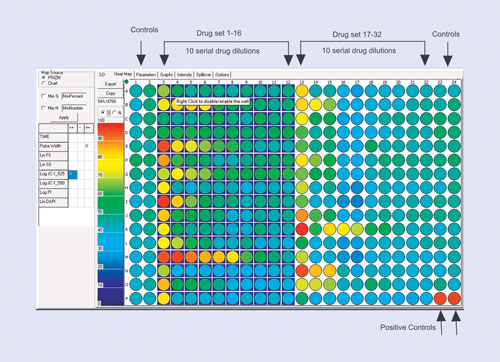
Figure 1. The plate layout for a 10-point dilution drug-screening study: Depending on the number of replicates, up to 32 drugs fit onto a 384-well plate with ease. The first two and last two columns are then defined as controls (although it is not necessary to use all these wells). The last two wells on the plate are used as a positive control. The scale on the left indicates either the total number of cells that respond or the percentage of cells in each well that respond.
The results are then analyzed by our software, which was created to provide entire plate-based results in what is essentially a single step directly from the flow-cytometry list-mode files. The creation of assay-specific, robust analytical tools to automate analysis is an innovation that transforms what would otherwise be a slow and cumbersome process of data reduction. Using such tools, we are able to reduce 384-well plate data to final results in about one minute—something traditional flow-cytometry software approaches cannot do.
To design an HT flow-cytometry system, one should have a well-defined result in mind, such as IC50 values when doing drug screens. Once this is established, it is possible to use a plug-and-play type interface to create the analysis protocol (Figure 2). Usually, the leading component is a gating region defining a specific subset of cells.
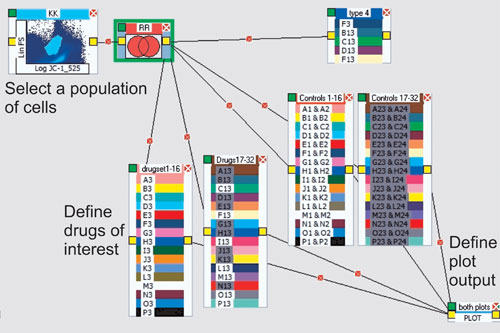
Figure 2. A method for creating an analysis protocol: A population is identified using two parameters (top left) and linked to a series of “drug containers” by “elastic lines.” These are subsequently joined to a plotting routine for production of the resulting IC50 curve. Each group of drugs can be plotted by simply clicking on that group. There is no restriction to the number of analytical processes that can be created using all of the tools available.
In the next step, a process icon such as a Boolean operator is identified, as well as a “container” that represents a layout to define the drug dilutions of interest. Finally, a plotting icon can be used to plot multiple containers on one graph. The final result is an IC50 curve and reported value as shown in Figure 3. IC50 plots are generated both visually and as XL spreadsheets that can be uploaded to any system. In fact, any result from any component can be visualized or delivered via spreadsheet files.
The process of creating an automated flow-cytometry screen is a comprehensive one. It requires appropriate design of the assay systems, creation of automated plate preparation, robotically managed sample collection, and, finally, a fast, very specific result-generation system. Traditional flow-cytometry analysis approaches are highly unlikely to be successful in dealing with the superabundance of data produced by HT systems.
Further, the concept of sequential analysis routinely used in flow-cytometry analysis simply fails when faced with the demand of large plate formats. This opens an opportunity to create relatively specific analytical tools that are designed to focus on multiple-well plates and to operate in a way that is fast and efficient.
So why use flow cytometry? A great majority of HT screening is performed on image-based instruments. This has become the standard across pharmaceutical companies and academia. However, it is time to rethink this logic. Flow cytometry has many advantages, particularly with suspended cells such as the HL60 cells used in the aforementioned assays. However, even attached cells can be successfully lifted from plates for flow cytometry.
A significant advantage of flow cytometry is that systems where multiple populations might exist, either physically or functionally, can be successfully managed, whereas imaging has great difficulty. Sample speed is generally much faster in flow cytometry than in imaging. A typical flow cytometry 384-well three- or four-color assay with 5,000 cells per well can easily be run in 10 minutes. It would take no longer to collect eight or nine fluorescent colors.
Collecting these numbers of cells and variables by imaging is well beyond even the fastest systems. No special plates are required for flow. The quality of the plate directly impacts the results in imaging, where minor variations in flatness or transmission can dramatically alter the results.
In flow, every cell is independently analyzed in the absence of all other cells. Imaging requires complex segmentation algorithms to separate cells and frequently the result is a compromise in identification. It is true that flow cytometry cannot provide the same type of morphological or location-specific data that imaging systems can, but the advantages flow cytometry brings for other analytical processes is very significant. High-speed flow cytometers matched with robotic delivery systems and robotic preparative systems are now an attractive alternative to current high-throughput microscopy-based systems. Speeding the analysis process has made high-throughput flow cytometry a transformational tool in the systems biology chest.
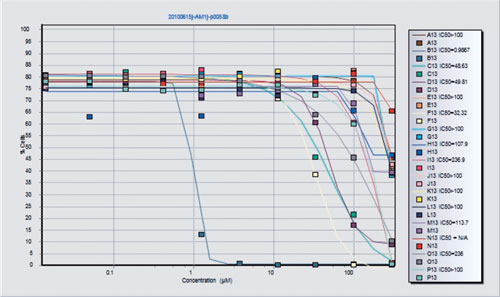
Figure 3. The final result for this assay is a direct IC50 readout from listmode files. This figure will be generated if the “plot” icon is clicked on Figure 2. Drug concentrations are shown on the x axis.
J. Paul Robinson, Ph.D. ([email protected]), is SVM professor of cytomics, professor of biomedical engineering, and director of the Purdue University Cytometry Laboratories. Valery Patsekin is a staff scientist at Purdue University, where Bartek Rajwa, Ph.D., is a professor of basic medical science, and V. Jo Davisson, Ph.D., is professor of medicinal chemistry and molecular pharmacology. Nianyu Li, Ph.D., and Padmakumar Narayanan, Ph.D., are scientists at Amgen.